Active symptomatic multiple myeloma (MM) is characterized by the presence of bone disease in the majority of patients. Advances in understanding the pathophysiology of osteolytic bone lesions in recent years have contributed to the development of therapeutic approaches that target both the tumor cells and the microenvironment. The bone provides a complex microenvironment where tumor and bone cells, such as osteoclasts (OC), osteoblasts (OB), and osteocytes, interact and induce bone destruction. This “bone niche” provides a permissive niche for the tumor cells and helps in the growth and propagation of MM cells. Therefore, targeting this niche has the potential of not only treating bone disease but also impacting long-term disease control.
Currently, bisphosphonates are the mainstay of therapy for MM bone disease. Nitrogen-containing bisphosphonates such as pamidronate (PAM) or zoledronic acid (ZA) reduce OC activity and affect OC survival in vitro and reduce skeletal-related events (SREs) in vivo.1 Recent data have also demonstrated a survival advantage with the use of ZA.1 Accordingly, guidelines for the treatment of MM-related bone disease recommend bisphosphonate use in all patients (with and without bone lesions).2 Either ZA or PAM is administered every three to four weeks during initial therapy in patients with active disease.2 Although these agents have proven to be very useful in mitigating SREs, they are accompanied by a significant risk of long-term toxicities such as osteonecrosis of the jaw.1 This has fueled the investigation of biomarker studies,3 prompting the use of bisphosphonates at a less frequent dosing schedule, as in the Z-MARK study.4
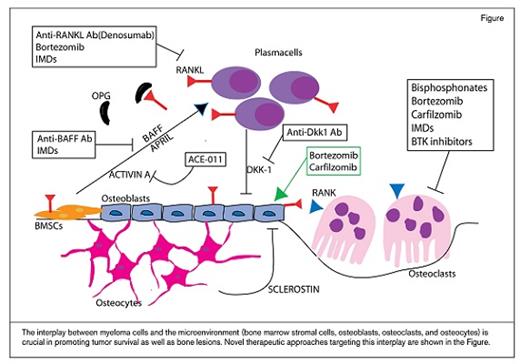
The interplay between myeloma cells and the microenvironment (bone marrow stromal cells, osteoblasts, osteoclasts, and osteocytes) is crucial in promoting tumor survival as well as bone lesions. Novel therapeutic approaches targeting this interplay are shown in the Figure.
The interplay between myeloma cells and the microenvironment (bone marrow stromal cells, osteoblasts, osteoclasts, and osteocytes) is crucial in promoting tumor survival as well as bone lesions. Novel therapeutic approaches targeting this interplay are shown in the Figure.
The interplay between myeloma cells and bone marrow stromal cells induces OC activity through the secretion of cytokines such as CCL3 (also known as macrophage inflammatory protein [MIP]-1α), MIP-1β, tumor necrosis factor-α, interleukin (IL)-3, IL-6, IL-11, stromal-derived factor-1 α, B-cell activating factor, activin A, and vascular endothelial growth factor.5 Many of these factors work through the receptor activator of nuclear factor kappa B (RANK), its ligand (RANKL), and its inhibitor osteoprotegerin (OPG).5 Therefore, the RANKL-OPG axis represents an important target in the treatment of MM bone disease. In a large randomized trial of patients with evidence of bone disease including MM, the anti-RANKL monoclonal antibody denosumab was not inferior to ZA and reduced the risk of SREs compared with ZA.1 There was, however, an increase in mortality in the MM subset, likely due to imbalances between the two arms.6 A larger international study (NCT01345019) is currently ongoing and will provide further evidence regarding the efficacy and safety of denosumab in MM.
Several other novel strategies have been studied in the context of targeting the OC. For example, Bruton’s tyrosine kinase (Btk) is a key kinase that plays a significant role in the development and function of normal B cells. Btk is highly expressed in MM cell lines and in plasma cells from MM patients. We have shown that Btk inhibition with CC-292 inhibits OC activity and improves MM bone disease. In preclinical studies, CC-292 has been combined with the proteasome inhibitor carfilzomib, and a synergistic effect in an MM xenograft mouse model was shown7 that warrants further investigation in clinical settings.
Anti-MM therapies also have a positive effect on the MM bone niche. Specifically, immunomodulatory drugs such as thalidomide or lenalidomide, approved and used for MM treatment because of their direct cytotoxicity against myeloma cells, have also been shown to inhibit angiogenesis and OC viability. They also play a role in inhibiting cytokines relevant to MM biology predisposing to OC activation. Proteasome inhibitors such as bortezomib and carfilzomib are also potent inhibitors of OCs.5 Therefore, treatment of the underlying MM is critical for continued improvement of MM-related bone disease.
Recent efforts have focused on understanding the role of the OB in myeloma bone diseases. Several cytokines and proteins regulating the OB axis have been discovered. In particular, wingless (Wnt) signaling pathway plays an important role in OB differentiation and in the interplay between MM cells and OB. The secreted glycoprotein Dickkopf-1 (Dkk-1), a soluble inhibitor of the Wnt signaling pathway, is expressed by OB and MM cells. Additionally, high levels of DKK1 have been found in patients with extensive bone disease.5 Promising preclinical data have demonstrated the potential use of anti-DKK1 therapy in clinical settings in MM. BHQ880, a human neutralizing IgG1 anti-DKK1 monoclonal antibody, was evaluated in a phase 1B clinical trial in combination with ZA in relapsed patients. The treatment was well tolerated, and beneficial effects were observed in some patients; however, no conclusion could be reached about the bone effect of BHQ880 because of the concomitant use of ZA and anti-MM therapy.8 High levels of sclerostin, another Wnt inhibitor, have been found in newly diagnosed MM patients, which correlates with MM disease stage and fractures. Encouraging preclinical data have highlighted the relevance of targeting sclerostin in MM.5 Similarly, activin A, a member of the transforming growth factor-β superfamily, stimulates OC differentiation and inhibits OB formation in MM. Following promising preclinical data with the soluble activin A receptor RAP-011, its human version ACE-011 was evaluated in a phase IIa study in patients with MM and has demonstrated reduced bone pain and cancer-induced anemia.5 Activin A neutralizing antibody in combination with lenalidomide has shown encouraging results in the preclinical setting,9 and the clinical trial evaluating ACE-011 with lenalidomide and dexamethasone (NCT01562405) is currently ongoing.
Proteasome inhibitors such as bortezomib, in addition to inhibiting MM–bone marrow stromal cell interactions and impairing osteoclastogenesis, also stimulates mesenchymal stem cell differentiation to OB and, therefore, actively modulates bone remodeling in MM.10 Data from clinical trials show that bortezomib significantly enhances markers of OB activity.5 The new proteasome inhibitor carfilzomib, besides its anti-tumor effect, has also demonstrated reversal of OB inhibition and improvement of bone disease in an in vivo MM mouse model.7
Despite considerable progress in the treatment of myeloma bone disease with anti-resorptive agents, this approach is largely palliative. More recent understanding of the OB axis provides new insights into MM-related bone biology and potential novel therapeutic approaches for treating bone disease. The interaction between MM cells and the OB, as well as the role of OB progenitors in the pathogenesis of MM bone disease, are areas of active investigation. Results from preclinical and clinical research seem to suggest eradication of myeloma and associated bone disease will require a combination of agents targeting tumor growth, osteoclastic bone resorption, and osteoblastic bone formation. Targeting the bone niche with these and other novel agents has the exciting potential of eradicating disease and preventing progression, with the goal of long-term disease control.
References
Competing Interests
Dr. Raje and Dr. Santo indicated no relevant conflicts of interest.