Two Mechanisms of Platelet Release from Bone Marrow into the Blood. The IL-1α-mediated megakaryocyte rupture mechanism on the left rapidly releases large numbers platelets into the marrow vascular sinus. The TPO-mediated pro-platelet formation mechanism on the right provides platelets at slower rates during steady-state conditions. From Nishimura S et al. J Cell Biol. 2015;209:453-466.
Two Mechanisms of Platelet Release from Bone Marrow into the Blood. The IL-1α-mediated megakaryocyte rupture mechanism on the left rapidly releases large numbers platelets into the marrow vascular sinus. The TPO-mediated pro-platelet formation mechanism on the right provides platelets at slower rates during steady-state conditions. From Nishimura S et al. J Cell Biol. 2015;209:453-466.
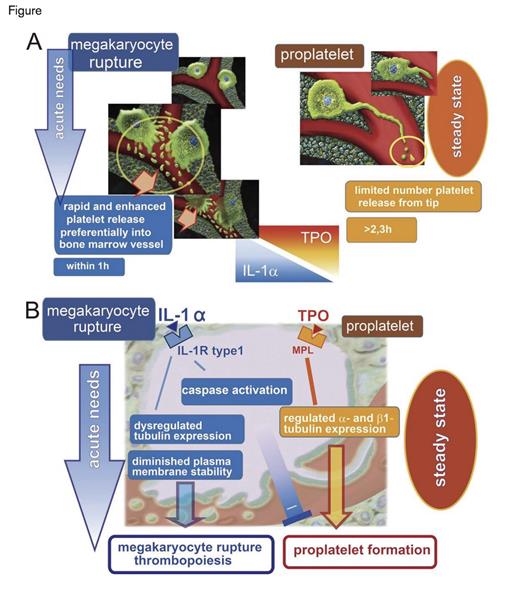
During periods of increased demand, production rates of specific blood cells increase. In the blood, reticulocytes increase after bleeding or hemolysis, and band-stage neutrophils increase with infection or inflammation. Reticulocytes and band-stage neutrophils reside temporarily in the marrow before entering the blood, but in addition to increased production rates, their release from the marrow is hastened with increased demand. Platelets, however, are produced by controlled fragmentation of megakaryocytes that remain in the marrow. Despite this difference in mechanism for producing terminally differentiated cells, a morphologically distinct population of platelets has been associated with increased rates of platelet production. Under conditions associated with increased demand, such as inflammation, blood loss, and recovery from thrombocytopenia, circulating platelets have increased size1 and RNA content.2 Under steady-state conditions, thrombopoietin (TPO) is the principal regulator of the development of megakaryocytes, which attain polyploidy, large size, and a position adjacent to marrow vascular sinuses.3 During steady-state platelet production, megakaryocytes form proplatelets, which are long, thin, branched, peripheral extensions created by the action of microtubule bundles (Figure).1 High concentrations of sphingosine 1-phosphate (S1P) in plasma relative to the marrow regulate formation and transendothelial extension of proplatelets into the sinusoidal lumen.4 S1P and shear forces created by blood flow cleave the terminal parts of proplatelets, forming single platelets or pre-platelets that are further cleaved, thus forming single platelets.4,5
Using multiphoton intravital microscopy of mouse cranium, Dr. Satoshi Nishimura and colleagues at the University of Tokyo report a new mechanism of platelet release into the circulation involving interleukin-1α (IL-1α) –mediated rupture of megakaryocytes that shower platelets into the marrow vascular sinus (Figure). The megakaryocyte rupture mechanism released slightly more than 40 platelets per megakaryocyte per minute into the blood, compared with the pro-platelet formation mechanism that released 1.4 and 2.6 platelets per megakaryocyte per minute at baseline and after TPO stimulation, respectively. Thioglycolate-induced peritoneal inflammation induced multiple inflammatory cytokines and increased platelets within a day in mice, but rapid immune-mediated reduction of platelet counts with anti-CD42 antibodies showed a specific increase in serum IL-1α, which peaked one day after administration. Serum TPO did not increase until a week after the induction of acute thrombocytopenia. Although IL-1α increased megakaryocyte number and polyploidy in vitro, the major difference compared with TPO was the mechanism of IL-1α–mediated platelet release into the blood. Exogenous IL-1α administered to normal mice (and confirmation of its specific effect with knockout mice) demonstrated that IL-1α rapidly increased platelets by promoting megakaryocyte rupture. When platelets newly released by IL-1α-induced megakaryocyte rupture were identified by their increased RNA content, they were more spherical, larger, and had decreased lifetimes compared with platelets of TPO-treated mice. Most importantly, however, IL-1α–induced platelets had aggregation and pro-thrombotic activities similar to platelets of TPO-treated mice.
Analyses of megakaryocytes in vitro demonstrated that IL-1α stimulation caused excessive β-tubulin accumulation without an accompanying α-tubulin increase. The resultant dysregulation of tubulin function is consistent with absence of pro-platelet formation in the megakaryocyte rupture mechanism. Further analyses of IL-1α–treated megakaryocytes showed activation of caspase-3 without the phenotype of apoptosis, but with a decreased membrane stability as shown by decreased mechanical stiffness and diminished ability to generate a mechanical force. Studies with knockout mice and treatment with inhibitors of microtubule assembly or caspase function confirmed that the megakaryocyte rupture mechanism of platelet release did not require TPO or normal microtubules, but it did require caspase-3.
In Brief
The IL-1α–induced megakaryocyte rupture mechanism of rapid platelet production described by Dr. Nishimura and colleagues helps explain elevated rates of platelet production during recovery from severe thrombocytopenia as well as elevated platelets in inflammatory states. The mechanism also explains increased platelet size associated with high platelet turnover. The way in which platelets produced by megakaryocyte rupture are preferentially delivered to the adjacent vascular sinus and how they cross the sinus endothelium are not clear. However, a better understanding of these aspects of the megakaryocyte rupture mechanism may provide treatments for thrombocytopenic patients. Administering an inducer of megakaryocyte rupture to patients with congenital thrombocytopenias or acquired thrombocytopenias, such as in myelodysplasia or aplasia, could increase platelet counts in acute bleeding episodes or before planned invasive procedures. Use of such a regulator of megakaryocyte rupture in a manner similar to the use of desmopressin in von Willebrand disease or mild Factor VIII deficiency has the potential to reduce platelet transfusions.
References
Competing Interests
Dr. Koury indicated no relevant conflicts of interest.