I grew up in a culture steeped in proverbs, where life events were often interpreted in allegorical context. My favorite saying was “It takes a village to raise a child,” because it aptly described my childhood in Lagos, Nigeria, growing up in a close-knit extended family. When I immigrated to the United States as a teenager, this adage took on richer meaning as my concept of family extended beyond blood relatives. My older brother became my guardian and closest confidante, when our undergraduate years overlapped at the University of California, Berkeley. My friends from the Berkeley African Students’ Association “adopted” me into their families, and we remain close to this day. In medical school at the University of California, San Francisco, my then boyfriend, now husband, bravely weathered the highs and lows of my early training. Stanford University has since served as the backdrop to many life and career milestones, spanning my internal medicine residency training to my current fourth year of hematology-oncology fellowship.
In the past two years, I have developed an even deeper appreciation for the village analogy as it pertains to my research career. My interest in sickle cell disease (SCD) started at an early age while I still lived in Nigeria, the country with the highest incidence of SCD in the world. Considered an orphan disease in the United States, annual SCD health-care costs approximate $1 billion because of the high acuity of care expended on its complications. One such resource-intensive condition is osteonecrosis of the femoral head (ONFH), which affects approximately 10 percent of all SCD patients. SCD-related ONFH is particularly morbid because of its rapid progression to femoral head collapse and the need for total hip arthroplasty at a relatively young age. There are no standards for care or effective pharmacologic management, and I felt this was an area of unmet clinical need and further investigation. My broad aim was to evaluate therapies that could alleviate ONFH symptoms and potentially reverse bone changes in affected SCD patients.
To optimize my exposure to SCD patients, Professor Stan Schrier wrote an introductory email on my behalf to his colleague, Dr. Elliott Vichinsky at UCSF Benioff Children’s Hospital Oakland, the largest SCD research institution in Northern California. Dr. Vichinsky introduced me to Drs. Carolyn Hoppe and Anne Marsh, who were exploring potential diagnostic biomarkers for ONFH in SCD patients. Thus began a rewarding collaboration and expansion of my mentoring team that gave me broad clinical exposure to SCD patients, while allowing me to cultivate my specific research interest in sickle bone disease.
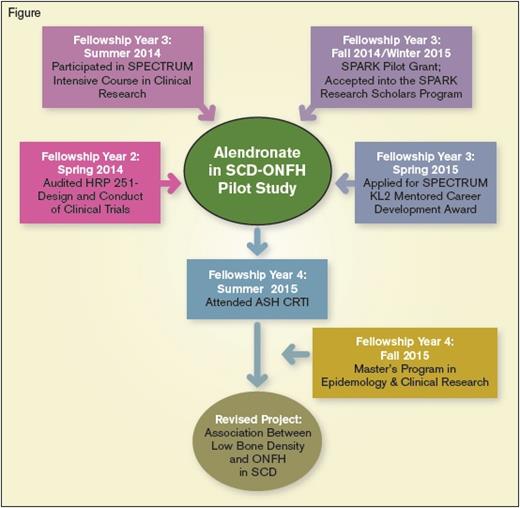
Dr. Hoppe’s mentorship was crucial to my application for a grant from the SPARK Translational Research Program at Stanford University. SPARK comprises a large group of academic researchers and industry leaders with drug development expertise, who are also committed to funding studies in orphan diseases. My proposal was a pilot clinical trial of the oral bisphosphonate alendronate in adolescent and adult SCD patients with ONFH (Figure). The primary endpoint was improvement in hip symptoms, defined as an increase of 15 points or greater from baseline on the Children’s Oakland Hip Evaluation Scale (CHOHES) – a validated clinical tool for SCD patients with ONFH. I then successfully applied for the NIH KL2 Mentored Career Development award from the Stanford Center for Clinical and Translational Research and Education (SPECTRUM). This grant covers my tuition for a Master’s degree in Epidemiology and Clinical Research at Stanford University, and provides protected time for my clinical trial endeavors. Through SPECTRUM, I obtained additional mentorship from Dr. Mary-Beth Leonard, a pediatric endocrinologist who studies bone complications in chronic childhood diseases. Dr. Leonard helped refine my research objectives by challenging me to clearly define the radiographic end points of my project and directing me to the available safety data on bisphosphonates in pediatric patients.
More recently, I participated in the 2015 ASH Clinical Research Training Institute (CRTI) for fellows and junior faculty interested in academia. My research goals were aligned with the SCD priorities set forth by ASH because I wanted to repurpose an existing drug, alendronate, to treat symptoms and retard the progression of SCD-related ONFH. I also wanted to evaluate the biomarkers investigated by Drs. Hoppe and Marsh, as potential predictors of treatment response. During the weeklong summer workshop, we received constructive criticism of our individual research proposals, presentation skills, and career development plans. The CRTI co-hosts, Drs. Sarah O’Brien and Joseph Mikhael worked tirelessly to ensure that all participants paired up with the most suitable CRTI faculty mentor, who would continue to work with us throughout the year.
My CRTI small group was composed of faculty mentors Drs. Adam Cuker, Jane Hankins, Anita Rajasekhar, Sara Vesely, and Lisbeth Welniak; and my co-participants Drs. Maissaa Janbain, Jacquelyn Powers, and Riten Kumar. We spent considerable time critiquing our clinical trial designs, brainstorming potential pitfalls, and strategizing methods to ensure successful implementation of our revised protocols. Although the group leaders found my proposed study of alendronate in SCD-related ONFH interesting, they felt it would be premature to conduct the trial without sufficient data on bisphosphonate safety in SCD patients. Since bisphosphonates are FDA approved for osteoporosis treatment, we conducted a thorough review of the SCD literature and found a relatively high prevalence of low bone density in the few published studies.1-3 We therefore redesigned my project as an observational study of the association between low bone density and SCD-related ONFH, which could potentially show preliminary data justifying an interventional study of alendronate to modify the natural history of OFNH. Dr. Hankins, my CRTI mentor, is a pediatric SCD expert at St. Jude Children’s Research Hospital, and she played a vital role in my protocol revision and final presentation. Dr. Hoppe connected us with her colleague Dr. Ellen Fung, who is a veteran investigator of bone metabolism and imaging in SCD and thalassemia at the Children’s Hospital Oakland Research Institute. My collaboration with Drs. Hankins and Fung will allow access to the extensive bone densitometry data on pediatric and adult SCD patients at their respective institutions, which will be crucial for the successful completion of my revised project.
I was inspired by the camaraderie among the faculty and my co-participants at the summer workshop, and I am looking forward to our reunion at the 57th ASH Annual Meeting in December. ASH CRTI has been a highlight of my fellow education; it embodies the “village” mentality of experienced elders guiding the younger generation to forge their place in society. While every fellow’s mentoring narrative is unique, I have come to appreciate the added value in constructing a mentoring network that not only embraces one’s home institution, but extends beyond it to include regional and national SCD expertise (Drs. Hoppe, Fung, and Hankins), different medical subspecialists (Dr. Mary Leonard), and the perspective of industry and biotech leaders (SPARK). CRTI consolidates these types of assets with a “deep bench” that provides many benefits to the junior investigator, not just during the year of dedicated mentorship, but throughout his/her entire career.
Mentor Testimony
“Serving as a mentor to Bimpe has been one of the most enjoying and fulfilling aspects of my career. As I have learned first-hand, mentorship is probably the single most important predictor of success for a junior investigator venturing into the increasingly challenging area of translational research. From Bimpe’s studies, we will obtain new and important insights into the risk factors and mechanisms involved in ONFH, as well as potential approaches to treat this understudied complication of SCD. It is a small investment to support Bimpe as she joins the future generation of physician-scientists and I am honored to be part of her ‘village’.” – Dr. Carolyn Hoppe, UCSF Benioff Children’s Hospital Oakland
“Bimpe is a hematologist/oncologist who, by embracing a career in adult SCD, is what I call a ‘rare bird.’ Her choice for studying ONFH reflects her perceptive eye for important understudied gaps in SCD. While promising, her study design lacked a strong scientific rationale for an intervention that, by treating bone mineral loss, would palliate ONFH. As commonly happens when proposals go through the critical, but constructive, eye of ASH CRTI, a step back is taken before a more ambitious study is launched. Her study is an example of this type of transformation. After our week at ASH CRTI, I continue to mentor her. I am fortunate to have met her and hope I can help bring her talents out in the open.” – Dr. Jane S. Hankins, St. Jude Children’s Research Hospital
References
Competing Interests
Dr. Gotlib, Editor-in-Chief of The Hematologist, serves as Director of the Stanford Hematology Fellowship Program and has also mentored Dr. Adesina.