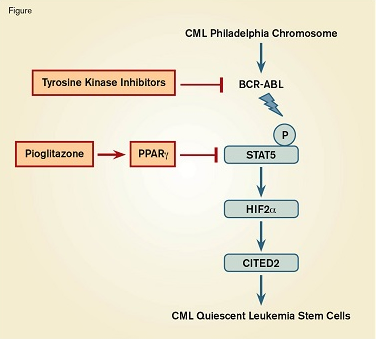
Targeted Combination Therapy to Eradicate Leukemia Stem Cells in Chronic Myeloid Leukemia (CML). The oncogenic BCR-ABL fusion protein phosphorylates and activates STAT5, which initiates a pathway leading to quiescence of leukemia stem cells. Tyrosine kinase inhibitors block BCR-ABL, whereas pioglitazone activates peroxisome proliferator-activated receptor gamma (PPARγ), which inhibits STAT5. The synergistic effect of both drugs on the quiescence pathway leads to the death of the leukemia stem cells.
Targeted Combination Therapy to Eradicate Leukemia Stem Cells in Chronic Myeloid Leukemia (CML). The oncogenic BCR-ABL fusion protein phosphorylates and activates STAT5, which initiates a pathway leading to quiescence of leukemia stem cells. Tyrosine kinase inhibitors block BCR-ABL, whereas pioglitazone activates peroxisome proliferator-activated receptor gamma (PPARγ), which inhibits STAT5. The synergistic effect of both drugs on the quiescence pathway leads to the death of the leukemia stem cells.
Chronic myeloid leukemia (CML) is a myeloproliferative disorder caused by a translocation between chromosomes 9 and 22 to form the Philadelphia chromosome. This encodes a BCR-ABL fusion protein and results in the constitutive activity of the oncogenic ABL tyrosine kinase. This leads to uncontrolled myeloid cell proliferation and CML, and without treatment, progresses to a fatal acute leukemia. With the advent of targeted chemotherapy in the form of tyrosine kinase inhibitors (TKIs), patients can go into long lasting remission and survival has improved dramatically. However, very few patients achieve a complete molecular response, since the TKIs fail to eradicate quiescent CML leukemia stem cells (LSCs), which can cause a relapse.
Dr. Stephane Prost and colleagues investigated the pathways regulating quiescence of LSCs. Prompted by the observation that the CML cell line K562 is sensitive to thiazolidinediones, they studied primary blood stem cells expressing CD34+ and the Philadelphia chromosome from chronic phase CML patients. Treatment of cells with a common TKI, imatinib, either alone or combined with pioglitazone (a thiazolidinedione derivative and an approved anti-diabetic drug), showed that pioglitazone purged quiescent LSCs, and that the two agents acted in synergy to deplete both proliferating and nonproliferating CML cells. Based on the knowledge that pioglitazone is a synthetic ligand that activates peroxisome proliferator-activated receptor gamma (PPARγ), the researchers investigated PPARγ to delineate its role in CML quiescence. PPARγ is a nuclear receptor protein that acts as a transcription factor to control the expression of several genes, including STAT5, which is down-regulated. The STAT5 A and B proteins are critical for normal stem cell maintenance and also play a role in CML since they are phosphorylated and activated by BCR-ABL kinase. STAT5 expression was elevated in CML and LSCs and exposure to pioglitazone markedly decreased STAT5 mRNA and protein levels, whereas imatinib prevented phosphorylation of the protein. Manipulating the levels of PPARγ and STAT5 by short interfering RNA or by overexpression following lentiviral transfer, confirmed the importance of the PPARγ –STAT5 pathway in LSCs. Further investigation of putative downstream transcriptional targets using similar molecular biology methods revealed the critical role of the transcription factor, hypoxia-inducible factor (HIF2α), and the transcription co-regulator CITED2, which is a master gene of stem cell quiescence.
Since pioglitazone is approved for clinical use in diabetic patients, the researchers tested the drug on three CML patients who still had residual disease despite imatinib treatment. Excitingly, all three patients achieved a complete molecular response and are still disease-free several years after discontinuing pioglitazone treatment. These findings prompted a phase II clinical trial, which produced very encouraging interim results.
In Brief
This study has provided important insights into the molecular pathways that regulate quiescence of leukemic stem cells in CML. Furthermore, the findings confirm the concept of “non-oncogene addiction,” whereby cancer cells are abnormally sensitive to fluctuations in a normal metabolic pathway, which may be exploited as a novel treatment option. These results also illustrate the exciting potential of repositioning drugs, already approved as safe to use for other diseases, to treat CML. This approach shortens the drug development pipeline and reduces the cost considerably. Finally, the advantages of combination therapy to erode the cancer stem cell pool are highlighted and provide hope for a cure for CML.
Competing Interests
Dr. Coetzer indicated no relevant conflicts of interest.