The topic of age-related clonal hematopoiesis in normal individuals emerged as a compelling story late in 2014 and dominated 2015 as a subject hematologists are still grappling to understand. It has become clear that in some individuals, these somatic clones might not always remain quiescent or harmless. Instead, they may contribute to clonal expansion and overt development of myeloid neoplasms with the acquisition of additional cooperating mutations.
One year ago, in the January/February 2015 Year’s Best issue of The Hematologist, Dr. Ravi Majeti authored the article titled “Pre-Leukemic Hematopoietic Stem Cells in Human Acute Myeloid Leukemia.” He highlighted the seminal findings, published concurrently by three groups,1-3 that some of the typical recurrent mutations found in acute myeloid leukemia (AML) could also be found in the peripheral blood of patients without a prior diagnosis of hematologic disease. Dr. Majeti raised the question of whether these individuals could be followed with the goal of detecting overt disease earlier, allowing for early treatment and/or prevention.
The studies by Dr. Siddhartha Jaiswal,1 Dr. Giulio Genovese,2 and Dr. Mingchao Xie3 and their respective colleagues established that these acquired mutations occurred with normal aging and were akin to precursor conditions such as monoclonal gammopathy of undetermined significance (MGUS) or monoclonal B-cell lymphocytosis (MBL). Analysis of mutations in 12,380 individuals in a Swedish study2 revealed that 10 percent of those older than 65 years had such mutations, compared with 1 percent younger than 50 years. The most frequent mutations in this study and in a similar study of 17,182 individuals in the United States and abroad1 were in the DNMT3A, ASXL1, and TET2 genes. Mutations in the genes JAK2, TP53, SF3B1, CBL, and SRSF2 were less common. Longitudinal follow-up of several cohorts revealed that individuals with clonal hematopoiesis exhibited an increased risk for development of hematologic cancers and all-cause mortality.
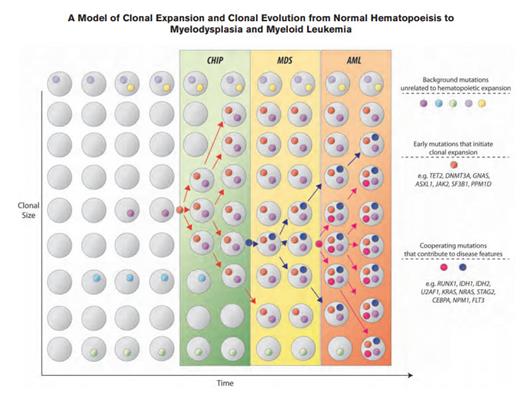
A Model of Clonal Expansion and Clonal Evolution From Normal Hematopoeisis to Myelodysplasia and Myeloid Leukemia. Clonal hematopoiesis of indeterminate potential (CHIP) as a precursor state for hematologic neoplasms. The figure depicts a model for evolution from normal hematopoiesis to CHIP and then, in some cases, to myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML). Hematopoietic progenitor cells or stem cells commonly acquire mutations throughout the human lifespan; most of these are passenger mutations that have no consequence for hematopoiesis. Certain mutations, however, confer a survival advantage to the mutated cell and its progeny and allow clonal expansion. Serial acquisition of mutations in an expanded clone can lead to a disease phenotype and ultimately, morbidity and mortality. CHIP can also directly progress to AML without an intervening MDS stage, and CHIP can progress to other conditions such as myeloproliferative neoplasms or lymphoid neoplasms. The majority of patients with CHIP will never develop an overt neoplasm, and patients will eventually die of unrelated causes.From Steensma et al. Blood. 2015;126:9-16, with permission.
A Model of Clonal Expansion and Clonal Evolution From Normal Hematopoeisis to Myelodysplasia and Myeloid Leukemia. Clonal hematopoiesis of indeterminate potential (CHIP) as a precursor state for hematologic neoplasms. The figure depicts a model for evolution from normal hematopoiesis to CHIP and then, in some cases, to myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML). Hematopoietic progenitor cells or stem cells commonly acquire mutations throughout the human lifespan; most of these are passenger mutations that have no consequence for hematopoiesis. Certain mutations, however, confer a survival advantage to the mutated cell and its progeny and allow clonal expansion. Serial acquisition of mutations in an expanded clone can lead to a disease phenotype and ultimately, morbidity and mortality. CHIP can also directly progress to AML without an intervening MDS stage, and CHIP can progress to other conditions such as myeloproliferative neoplasms or lymphoid neoplasms. The majority of patients with CHIP will never develop an overt neoplasm, and patients will eventually die of unrelated causes.From Steensma et al. Blood. 2015;126:9-16, with permission.
In 2015, we saw that these “seeds” could remain dormant, in a state of “clonal hematopoiesis of indeterminate potential (CHIP).”4 CHIP is defined as at least 2 percent variant allele frequency (VAF) of somatic mutations known to be associated with hematopoietic neoplasms, but without fulfilling diagnostic criteria for the malignancy (e.g., MDS or AML).4 There is a 0.5 to 1 percent risk of progression to overt malignancy per year (Figure), with increased rates in patients with a higher VAF of the somatic mutation.
Instead of remaining dormant, such clones may demonstrate opportunistic tendencies. In patients undergoing chemotherapy, clones may expand under the selective pressure of treatment and contribute to the development of therapy-related MDS or AML (t-MDS/t-AML).5 Historically, it was believed that chemotherapy and radiation led to therapy-related hematologic cancers because of increased numbers of mutations induced by treatment. However, contrary to this dogma, Dr. Terrence Wong and colleagues’ analysis5 of t-AML patient samples showed that there was not an increased number of mutations compared with de novo AML. Moreover, the same AML-associated mutations could be detected long before the diagnosis of cancer, and resistant clones simply expanded after treatment of the cancer.5 For example, in one patient with Hodgkin lymphoma, two TP53 mutations were detected in leukapheresis samples at a VAF of 0.5 percent six years prior to development of t-AML, and both TET2 and NUP98 mutations subsequently arose as potential driver mutations, only detectable later at the time when the leukemia was diagnosed.
Notably, two of the three most commonly observed mutated genes in normal individuals, DNMT3A and TET2, were also found to be the most frequent mutations that failed to clear by day 30 after induction chemotherapy for AML. Patients in whom these mutations persist exhibit a worse outcome, even in complete remission.6 Clearly, these mutations are not always innocuous. It will be our task as hematologists to contextualize the prognostic importance of these molecular abnormalities, not only patients with MDS or AML, but also in individuals with mild cytopenias or dysplasia of unknown significance (ICUS or IDUS, respectively) who do not meet diagnostic criteria for MDS. And what do we tell the otherwise normal individual who is referred to our clinic with one or more “hits” uncovered on a myeloid gene panel that should not have been ordered in the first place? We have much to learn ourselves before we can confidently guide patients through this maze of genetic complexity.
References
Competing Interests
Dr. Becker indicated no relevant conflicts of interest.