Sideroblastic anemias are a group of congenital and acquired bone marrow disorders with varied underlying pathophysiologies, primarily related to a heme or hemoglobin synthesis deficiency, and characterized by pathologic iron deposits in the mitochondria of erythroid precursors. Among the congenital sideroblastic anemias, the most common form is X-linked sideroblastic anemia due to mutations in δ-aminolevulinate synthase 2 (ALAS2). ALAS2 is the first enzyme in the heme synthetic pathway; it catalyzes the formation of δ-aminolevulinate (ALA) from glycine and succinyl-CoA and is an erythroid-specific enzyme. Most pathogenic mutations are partial loss-of-function alleles.1
Dr. Duane L. Guernsey and colleagues previously demonstrated that mutations in the erythroid-specific inner mitochondrial membrane transporter, SLC25A38, cause an autosomal recessive form of congenital sideroblastic anemia.2 SLC25A38 is highly and preferentially expressed in transferrin receptor positive erythroid cells and deletion of the SLC25A38 homologue in yeast causes reduced levels of δ- aminolevulinate and a defect in heme synthesis. The defect in heme synthesis can be rescued by supplementing the media with ALA or glycine. Dr. Guernsey and colleagues speculated that SLC25A38 facilitates ALA production by importing glycine into the mitochondria or by exchanging glycine for ALA across the inner mitochondrial membrane. However, the precise function of SLC25A38 remained unknown.
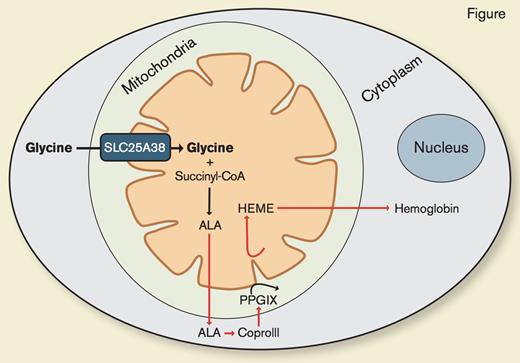
SLC25A38 Is a Mitochondrial Glycine Transporter Required for Heme Synthesis. Adapted from Figure S6 from Fernández-Murray JP et al. PLoS Genet. 2016;12:e1005783.
SLC25A38 Is a Mitochondrial Glycine Transporter Required for Heme Synthesis. Adapted from Figure S6 from Fernández-Murray JP et al. PLoS Genet. 2016;12:e1005783.
To determine the function of SLC25A38 and to identify potential therapies for what is emerging as a relatively common form of congenital sideroblastic anemia,3 Dr. J. Pedro Fernández-Murray and colleagues also used yeast-deletional strains and a morpholino-knockout zebrafish model. As previously shown,2 they found that yeast cells lacking the yeast homologue of SLC25A38 exhibit decreased heme levels. Heme synthesis was restored with expression of the human SLC25A38 protein or with ALA or glycine supplementation. They went on to establish that SLC25A38 functions as a mitochondrial glycine importer, and that cytoplasmic glycine is the main source of glycine for heme synthesis within the mitochondria (Figure). Additionally, they replicated earlier work2 showing that morpholino knockdown of both zebrafish homologues of SLC25A38 results in anemia. They demonstrated that neither ALA nor glycine supplementation in the zebrafish model could ameliorate the anemia, in contrast to their yeast model where this supplementation restored heme synthesis. To account for this finding, the authors cleverly recognized that while yeast have the capacity to synthesize folate, which is needed for cell growth, vertebrates such as zebrafish and humans require dietary folate supplementation, providing the rationale to treat the knockout morphants with glycine and folate. This combination improved hemoglobinization to near normal levels.
In Brief
In humans, congenital sideroblastic anemia due to SLC25A38 deficiency typically presents as a transfusion-dependent anemia, and thus, patients are faced with the burden and complications of a lifelong, chronic transfusion program. The elegant biology carefully deciphered in this paper identifies glycine and folic acid supplementation as a simple therapy for this form of congenital sideroblastic anemia. Confirmation of its safety and efficacy in patients awaits further study.
References
Competing Interests
Dr. Keel indicated no relevant conflicts of interest.