Morphologically, Hodgkin lymphoma (HL) is characterized by rare malignant Reed-Sternberg (RS) cells surrounded by an ineffective infiltrate of inflammatory and immune cells. Using checkpoint inhibitors to redirect the immune system to eliminate RS cells is an attractive therapeutic approach. Nivolumab and pembrolizumab, the monoclonal antibodies directed against PD-1, demonstrated dramatic activity in phase I studies, with the vast majority of patients with refractory disease responding to therapy. In their recent analysis of tissue biopsies from patients with HL, Dr. Margaretha G.M. Roemer and colleagues elucidate the biologic basis for the impressive activity of immunotherapy in this disease.
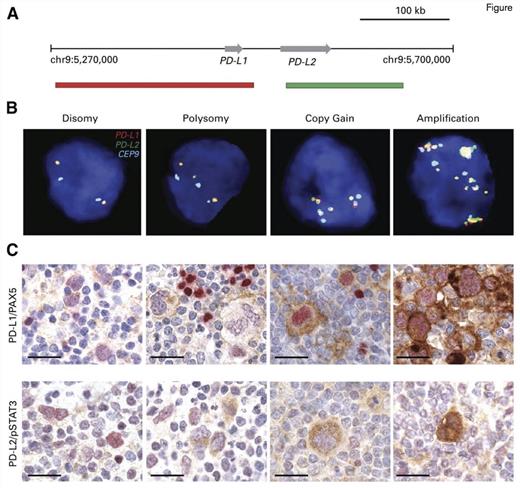
"Genetic and Immunohistochemical Analyses of the PD-L1 and PD-L2 Loci and PD-1 Ligand Expression. (A) Location and color labeling of the bacterial artificial chromosome (chr) clones on 9p24.1 used for fluorescent in situ hybridization (FISH). RP11-599H20 including PD-L1, labeled red. RP11-635N21 including PD-L2, labeled green. (B) Representative images of FISH results for the various categories. PD-L1 in red, PD-L2 in green, fused (F) signals in yellow, and centromeric probe in aqua (A). In these images, disomy reflects 2A:2F; polysomy, 3A:3F; copy gain, 3A:6F; and amplification, 15+F. (C) The top panel shows PD-L1 (brown)/PAX5 (red) immunohistochemistry (IHC) in the classical Hodgkin lymphoma cases with 9p24.1 disomy, polysomy, copy gain, and amplification from (B). The bottom panel shows PD-L2 (brown)/pSTAT3 (red) IHC in the same cHL cases. Scale bar = 50 μm.
"Genetic and Immunohistochemical Analyses of the PD-L1 and PD-L2 Loci and PD-1 Ligand Expression. (A) Location and color labeling of the bacterial artificial chromosome (chr) clones on 9p24.1 used for fluorescent in situ hybridization (FISH). RP11-599H20 including PD-L1, labeled red. RP11-635N21 including PD-L2, labeled green. (B) Representative images of FISH results for the various categories. PD-L1 in red, PD-L2 in green, fused (F) signals in yellow, and centromeric probe in aqua (A). In these images, disomy reflects 2A:2F; polysomy, 3A:3F; copy gain, 3A:6F; and amplification, 15+F. (C) The top panel shows PD-L1 (brown)/PAX5 (red) immunohistochemistry (IHC) in the classical Hodgkin lymphoma cases with 9p24.1 disomy, polysomy, copy gain, and amplification from (B). The bottom panel shows PD-L2 (brown)/pSTAT3 (red) IHC in the same cHL cases. Scale bar = 50 μm.
The investigators analyzed a cohort of 108 HL patients, all of whom were treated with the Stanford V regimen. The median age was 30 years, and the majority of patients had the nodular sclerosis subtype of the disease. Thirty-three and 41 patients had early-stage favorable (as defined by lack of B symptoms or disease bulk) and unfavorable disease, respectively. Thirty-four patients had advanced-stage disease. Using fluorescence in situ hybridization, researchers assessed alterations in chromosome 9p24, which encodes the programmed death 1(PD-1) ligands PDL-1 and PDL-2, as well as JAK2 and related transducers and activators of JAK-STAT signaling. Additionally, given the known increased expression of PD-1 ligands in Epstein-Barr virus–positive HL, EBV-encoded RNA (EBER) status was determined. Approximately 50 RS cells per case were evaluated. Compared with the control signal, cases with a 1:1 ratio were categorized as disomic (n=1), and those with a ratio of 1:1 but with more than two copies of each of the PDL-1 and PDL-2 probes were considered polysomic (n=5). Ratios greater than 1:1 but less than 1:3 were labeled as copy gain (n=61) and those cases with greater than 3:1 were classified as amplified (n=39). Two cases harbored translocations. The highest alteration in 9p24 for each case defined the category. Furthermore, the expression of PD-1 ligands was evaluated using immunohistochemical staining (Figure). All cases were concordant for alterations in PD-L1 and PD-L2. Altogether, 107 of 108 cases harbored alterations in 9p24.
The authors correlated clinical risk and genetic alterations with progression-free survival (PFS). As expected, patients with early-stage disease, both with favorable and unfavorable risk profiles, experienced excellent PFS. Patients with advanced-stage disease, however, were more likely to develop recurrent disease. Importantly, outcome was stratified by copy number alterations of 9p24. No relapses occurred in patients with polysomy. The highest relapse rate was seen in those with amplification, and intermediate PFS was observed in the group with copy gains. Additionally, patients with advanced-stage disease were more likely to harbor amplification compared to those with early-stage disease. On multivariate analysis, only stage was predictive of PFS, with a trend for amplification (p=0.075).
In Brief
These findings elucidate the mechanism underlying the remarkable clinical activity of nivolumab and pembrolizumab in patients with classical HL. Selecting patients with higher risk disease at baseline based on copy number gain, in addition to clinical risk factors, may be important in identifying those patients most likely to benefit from these agents. In turn, this may facilitate further reductions in the use of chemotherapy and radiation, which are associated with late effects in this group of patients who typically experience long term survival.
Competing Interests
Dr. LaCasce indicated no relevant conflicts of interest.