Hematology research has become increasingly focused on the study of cellular heterogeneity and parsing out the biologic roles of specific cells. For example, such investigations may explore novel T cell subsets mediating immune responses, distinguish pre-leukemic cells from healthy ones, discover the various roles of uni- or multipotent progenitors to the overall contribution of blood production, and identify the specific stromal cells that govern normal or malignant niches. Early on, the microscope was one of the only tools researchers had to study different cells; it was used to make observations regarding cell morphology, nuclear complexity, and differential staining to basic or acidic dyes. The armamentarium has expanded remarkably since then, with a vast array of antibodies that identify unique markers on cells. The tools even allow for flow cytometric techniques such as CyTOF that can identify dozens of markers on a single cell; for genetic mice with gene-specific reporter systems, or for gene-specific Cre recombinase allowing for deletion; to live in vivo imaging permitting for the direct observation of cells within their natural environment.
The development of these observational tools has also led to the creation of methods to specifically delete a population of cells in a mouse, allowing a researcher to determine specific biologic functions of that cell population in an intact organism. Infusion of an anti-CD3 antibody to remove T cells can be an effective method of cell ablation. However, this requires a unique surface antigen as well as antibodies with predictable pharmacokinetics and in vivo activity. Sometimes the deletion method is as simple as deleting a specific gene which is unique and necessary for a population of cells. Nevertheless, not every cell type can be deleted in this way, and often there are issues with various genes being ubiquitously expressed during embryogenesis, necessitating floxed alleles and more complicated Cre systems. To allow for cell specific ablation with temporal control, many researchers develop mice in which a cell-specific promoter region drives expression of the diphtheria toxin receptor. When mice are injected with the toxin, the cell type of interest is killed. In many cases, this alleviates concerns over differences in embryonic versus adult organisms and allows for timed ablation of a cell. However, diphtheria toxin administration and death of cells by this manner can cause significant non-specific effects in the mice. In the blood system, both the diphtheria toxin approach and antibodies also have the disadvantage of needing repeated administrations as the cells are rapidly reconstituted.
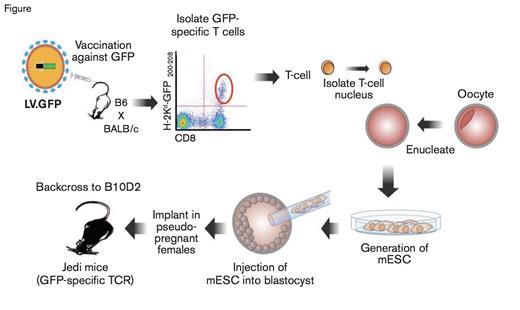
Schematic of the Methodology to Produce Jedi Mice. EGFP, enhanced green fluorescent protein; LV.EGFP, lentivirus expressing EGFP (LV.EGFP); TCR, T cell receptor.
Schematic of the Methodology to Produce Jedi Mice. EGFP, enhanced green fluorescent protein; LV.EGFP, lentivirus expressing EGFP (LV.EGFP); TCR, T cell receptor.
Recent work from Dr. Judith Agudo and colleagues now adds another arrow in the quiver of cell ablation techniques that may have unique applicability to the field of hematology. They reasoned that T cell mediated cell ablation would allow for not only cell specificity, but also for a single administration with sustained effects, unlike antibodies or diphtheria toxin. However, making a cohort of T cells for each cell type of interest would not be very practical, so the researchers cleverly chose to generate T cells with a receptor capable of recognizing EGFP-specific MHC I expression. To generate these EGFP specific T cells, the authors immunized mice with a lentivirus expressing EGFP, and isolated EGFP specific T cells (Figure). These cells were then used as a nuclear donor for somatic cell nuclear transfer. The process created mice that already have the rearranged T cell receptor specific for EGFP, preventing the need for continuous culture of T cell clones. These mice now produce what the authors termed just EGFP death-inducing (Jedi) T cells, that will destroy any cell expressing EGFP.
To test these T cells in in vivo systems, the authors used three separate models. First, they used the readily available CX3CR1-EGFP mice, which express EGFP in macrophage lineages, including microglia. When Jedi T cells were transferred into these mice, within one week of infusion, all of the microglia were eliminated, which suggests the ability of Jedi T cells to cross the blood brain barrier. However, since EGFP is expressed on macrophages, prior to performing the experiment, the authors irradiated the mice and transplanted them with bone marrow from RFP expressing mice in an effort to restrict EGFP expression to only microglia. Therefore, as the authors state in their manuscript, the effects of irradiation in this model on the integrity of the blood brain barrier cannot be ruled out. However, these studies do demonstrate a remarkable ability for efficient and sustained cell ablation within the brain. The authors then used a Foxp3-EGFP mouse, where regulatory T cells express EGFP, and infused them with Jedi T cells to eliminate Tregs. These studies showed robust ablation of the cells, with phenotypes consistent with reports of Foxp3 cell deletion. Finally, to demonstrate the ability to delete a rare population of cells, the authors utilized a hyperpolarization-activated cyclic nucleotide-gated channel (Hcn) EGFP mouse. These cells comprise less than 10,000 of the cells in the heart. Within 10 days of infusion, all of the Hcn+ cells were eliminated, and since they express EGFP, this ablation could be directly visualized and assessed by flow cytometry.
In Brief
The Jedi mouse could be a remarkable tool to advance hematology research. One caveat to this model is that the recognition is restricted to H-2KD allele, necessitating backcrossing of existing C57Bl/6 strains to either DBA or B10D2 mice. For transplantation studies, or mice with complicated genetics, this could be a barrier to use. Since the cytotoxic T-cell–mediated killing is a natural response of these cells, it is likely that there will be reduced off-target toxicities as compared to diphtheria toxin approaches. Given the abundance of already existing reporter mouse strains that express EGFP, the Jedi mice and their T cells represent a potentially powerful tool for further cell-specific ablation in hematologic research, supporting studies on the hematopoietic niche, as well as cell-specific immune effects. With the ease of adding EGFP to cancer cell lines, one can envision using these as a method to study T-cell–mediated immune surveillance, or even models of minimal residual disease by tracking remaining EGFP expressing cells after Jedi T-cell infusion.
Competing Interests
Dr. Hoggatt indicated no relevant conflicts of interest.