The Question
What is your diagnostic approach to monocytosis?
Case
A 73-year old woman has had monocytosis for at least seven years. She has a history significant for rheumatoid arthritis and anemia of chronic disease. In 2010, her white blood cell count was 9.8 × 109/L with 59 percent neutrophils, 19 percent lymphocytes, and 21 percent monocytes (absolute monocyte count 2.1 × 109/L). Her red blood cell count was 3.2 × 1012/L, hemoglobin was 10.0 g/dL, mean corpuscular volume was 91.5 fL, and platelets were 162 × 109/L. The peripheral smear did not show immature monocytes. A bone marrow biopsy performed at that time was normocellular for age (40%). Significant findings were limited to increased mature monocytes (12% by manual differential) and mild dyserythropoiesis (<10%). Flow cytometry showed no increase in blasts (0.3% CD34 positive events), polytypic B-cells, and phenotypically normal T-cells. A concurrent unstimulated karyotype was normal. Six months later, the absolute monocyte count remained elevated at 1.3 × 109/L, and a repeat bone marrow biopsy with flow cytometry and cytogenetics reported similar results.
Our Response
Examining the Blood Smear
Peripheral smear review is essential in cases of sustained monocytosis because morphology is critical to correct classification and diagnostic testing recommendations. Morphology is required to assess maturity in monocytes and to distinguish between monocytes and promonocytes. This difference is crucial since promonocytes are blast equivalents and should be included in the blast count. Additionally, assessment for dysplasia is required, as this provides evidence for hematologic malignancy. This is especially important in the assessment of neutrophils for dysplastic features, such as nuclear hyposegmentation and hypogranular cytoplasm. In the setting of bone marrow stress, rheumatoid arthritis, and other autoimmune disorders, dyserythropoiesis in blood may be observed, so assessment of red blood cells is not as useful as neutrophil evaluation.1 If clinical correlation fails to explain a case of a sustained peripheral blood monocytosis, a bone marrow specimen (aspirate and biopsy) with cytogenetic karyotyping is indicated.
Reactive Monocytes
The differential diagnosis for reactive monocytosis is broad. Infection is the most common cause of reactive monocytosis. Other common causes of monocytosis include inflammatory conditions such as collagen vascular disease, chronic neutropenia, splenectomy, hemolytic anemia, immune thrombocytopenic purpura, and both hematopoietic and nonhematopoietic neoplasms.
Flow cytometry is capable of unveiling subtle immunophenotypic aberrancies, but it is not able to reliably distinguish promonocytes (blast equivalents) or monoblasts from mature monocytes. Because of this, flow cytometry is the least reliable modality to assess for a neoplastic monocytic disease. Reactive monocytes may have aberrant expression by flow cytometry, particularly CD56 and/or diminished human leukocyte antigen–DR, CD33, and CD13. The finding of two or more aberrancies favors a neoplastic process, though this is not specific.2 Recently, it was reported that a high fraction of CD14+/CD16– onocytes distinguishes chronic myelomonocytic leukemia (CMML) from reactive causes.3
Neoplastic Monocytes
Several primary hematologic neoplasms harbor neoplastic monocytes including CMML, acute myeloid leukemia with monocytic differentiation, and myeloproliferative neoplasms such as chronic myelogenous leukemia (CML). While the peripheral smear of CML is usually characteristic, the findings of CMML and acute leukemia with monocytic differentiation may be similar in peripheral blood. The bone marrow is often the site of increased immature monocytes. For this reason, bone marrow examination is essential when a neoplastic process with monocytic differentiation is suspected.
Morphology is critical to enumerating monoblasts and the blast-equivalent promonocytes, and to distinguishing them from mature monocytes, both reactive and dysplastic. As already mentioned, this distinction is not possible by flow cytometry. Cytochemical staining may be useful since it improves the sensitivity over morphology alone for the detection of monocytic cells.4
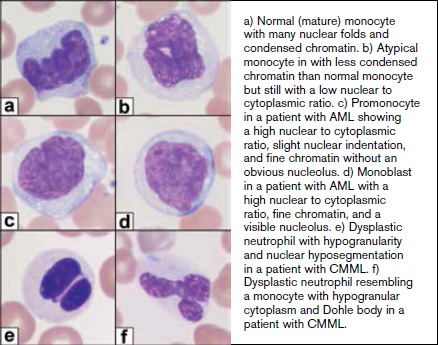
a) Normal (mature) monocyte with many nuclear folds and condensed chromatin. b) Atypical monocyte with less condensed chromatin than normal monocyte but still with a low nuclear to cytoplasmic ratio. c) Promonocyte in a patient with AML showing a high nuclear to cytoplasmic ratio, slight nuclear indentation, and fine chromatin without an obvious nucleolus. d) Monoblast in a patient with AML with a high nuclear to cytoplasmic ratio, fine chromatin, and a visible nucleolus. e) Dysplastic neutrophil with hypogranularity and nuclear hyposegmentation in a patient with CMML. f) Dysplastic neutrophil resembling a monocyte with hypogranular cytoplasm and Döhle body in a patient with CMML.
a) Normal (mature) monocyte with many nuclear folds and condensed chromatin. b) Atypical monocyte with less condensed chromatin than normal monocyte but still with a low nuclear to cytoplasmic ratio. c) Promonocyte in a patient with AML showing a high nuclear to cytoplasmic ratio, slight nuclear indentation, and fine chromatin without an obvious nucleolus. d) Monoblast in a patient with AML with a high nuclear to cytoplasmic ratio, fine chromatin, and a visible nucleolus. e) Dysplastic neutrophil with hypogranularity and nuclear hyposegmentation in a patient with CMML. f) Dysplastic neutrophil resembling a monocyte with hypogranular cytoplasm and Döhle body in a patient with CMML.
Based on World Health Organization (WHO) 2008 criteria, the diagnosis of CMML requires at least three months of monocytosis (>1.0 × 109/L) with dysplasia in at least one myeloid lineage. When dysplasia is absent, the presence of an acquired cytogenetic or molecular clone is required. Other disease-defining translocations, notably BCR-ABL1 fusion, must not be present. In the setting of CMML, one must also be on the lookout for systemic mastocytosis, which may be seen concurrently. CMML is the myeloid malignancy most frequently described in this setting. In the upcoming revision to the 4th edition of the WHO, further subtypes of CMML with prognostic significance will be acknowledged. Based on a WBC above or below 13 x 109/L, a proliferative and dysplastic subtype can be assigned, respectively. Additionally, CMML-0 will be introduced for cases in which the blast count is less than 2% in the peripheral blood and 5% in the bone marrow.5
Cytogenetic abnormalities are seen in a minority of cases of CMML (approximately 30%). The detection of a clonal cytogenetic abnormality, with few exceptions, confirms a neoplastic process. In contrast, a positive molecular result does not confirm a neoplastic process, since mutations are detected in normal subjects. Mutations in confirmed cases of CMML are found in nearly all cases.6 Many somatic mutations have been described in CMML, although none are specific to the disease. Some of the most common mutations are TET2, ASXL1, SRSF2, and SETBP1. In some studies, SRSF2 is the most common mutation, and it is most often seen in the setting of a normal karyotype.7 SETBP1 and ASXL1 are associated with reduced overall survival.6,8 RUNX1 has been associated with a worse prognosis.9 NPM1 mutations have been linked to a high risk of transformation to AML.10 Interestingly, recent literature has shown that approximately 10 percent of individuals older than 70 years harbor a mutation in peripheral blood, many of which are seen in CMML, including ASXL1 and TET2.11 This finding shows that, in contrast to cytogenetic abnormalities, the detection of an isolated molecular abnormality in a case of monocytosis does not necessarily confirm a neoplastic diagnosis.
The incidence of transformation from CMML to AML is variable, ranging from 15 to 52 percent.12 Sequential karyotyping has prognostic value, in that approximately 25 percent of CMML patients will acquire cytogenetic abnormalities not present at diagnosis. Not surprisingly, the development of a complex karyotype is associated with progression to AML. Those with the addition of del(20q) tend to have stable disease.13
Patient Follow-up
In this patient, the absolute monocyte count has fluctuated but has remained elevated since at least 2010, ranging from 0.9 × 109/L to 3.2 × 109/L. Platelet counts have remained mildly decreased (102 × 109/L - 137 × 109/L), and anemia has never been present. In 2015, the absolute monocyte count was 3.2 × 109/L which triggered a flow cytometry study on peripheral blood. This showed 62 percent monocytes with partial expression of CD56, but without additional immunophenotypic abnormality. A subsequent CBC several months later showed a stable monocyte count of 3.1 × 109/L.
Overall, the findings in this case are consistent with a reactive monocytosis. In rheumatoid arthritis, as in other autoimmune disease, monocytosis is seen in a significant minority of patients and is typically mild.14 The monocytosis has been stable, and significant dysplasia is not present in the peripheral blood or bone marrow. Repeat bone marrow biopsy with karyotype failed to reveal evidence of a neoplastic process. In this setting, pursuing molecular studies to detect a mutation is not clearly indicated and may even lead to an erroneous diagnosis. Given the relatively high prevalence of mutations in this population (e.g., clonal hematopoiesis of indeterminate potential), the finding of one of these mutations would not be diagnostic of a myeloid neoplasm, and could rather represent age-related clonal hematopoiesis.11 However, the finding of a subsequent cytogenetic abnormality would be of great value in making a diagnosis, and this could be potentially performed on peripheral blood.
Monocytosis is both a common and challenging finding. Accurate diagnosis of the many entities which present as monocytosis requires the integration of clinical history, morphology, and the judicious use of ancillary studies.
References
Competing Interests
Dr. Lynch and Dr. Foucar indicated no relevant conflicts of interest.