For decades, there has been considerable interest in understanding the molecular mechanisms governing hematopoietic stem cell (HSC) self-renewal versus differentiation. In particular, the concept of maintaining “stemness” during ex vivo culture conditions has been attractive to researchers hoping to expand smaller sources of HSCs for transplantation, such as those acquired from umbilical cord blood, or for those researchers interested in genetically manipulating HSCs for curative therapies of genetic disorders.
Several different experimental systems have been used to identify regulators of HSC self-renewal, some of which have advanced to early therapeutic interventions in transplantation. One approach has been the use of small molecule chemical screens, either on nonhuman cells like zebrafish embryos or on primary human CD34+ cells. These screens have identified molecules such as prostaglandin E2, UM171, or the aryl hydrocarbon receptor (AHR) antagonist SR1.1 Other studies have used genetic approaches in mice like RNAi screens, retroviral insertion screens, or hypothesis driven genetic alteration of HSCs. Intriguingly, using all of these methods, several groups have previously reported2-5 that the RNA binding protein Musashi-2, originally discovered in Drosophila as a regulator of asymmetric cell division, is a key regulator of murine hematopoiesis. The mechanisms governing Musashi-2 regulation, and the exact hematopoietic effects, have been mixed depending on the mouse models used and assays performed.
A recent report by Dr. Stefan Rentas and colleagues sought to characterize the role of Musashi-2 overexpression in regulating human HSCs and to develop a mechanistic understanding of Musashi-2 regulation of hematopoiesis. They demonstrated that lentiviral overexpression of Musashi-2 enhanced the number of human short-term repopulating cells, as demonstrated in xenotransplantation assays in mice, as well as a suggestion of enhanced long-term repopulating HSCs after ex vivo culture. The authors then performed RNA sequencing of the Musashi-2 overexpressing CD34+ cells and of CD34+ cells with a lentiviral shRNA knockdown of Musashi-2. Surprisingly, pathway analysis revealed that targets involved in AHR signaling were suppressed in Musashi-2 overexpressing cells, while the inverse was true after Musashi-2 knockdown. Given that the AHR is the same target of SR1, which is currently being clinically explored for HSC expansion,6 the authors performed gene set enrichment analysis (GSEA) and showed that genes that were downregulated by Musashi-2 overexpression significantly matched those downregulated with SR1 treatment.
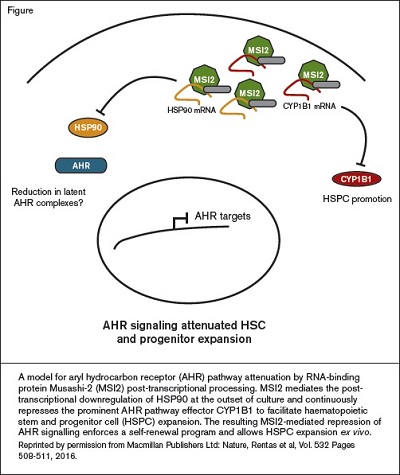
A model for aryl hydrocarbon receptor (AHR) pathway attenuation by RNA-binding protein Musashi-2 (MSI2) post-transcriptional processing. MSI2 mediates the posttranscriptional downregulation of HSP90 at the outset of culture and continuously represses the prominent AHR pathway effector CYP1B1 to facilitate haematopoietic stem and progenitor cell (HSPC) expansion. The resulting MSI2-mediated repression of AHR signalling enforces a self-renewal program and allows HSPC expansion ex vivo. Reprinted by permission from Macmillan Publishers Ltd: Nature, Rentas et al, Vol. 532 Pages 508-511, 2016.
A model for aryl hydrocarbon receptor (AHR) pathway attenuation by RNA-binding protein Musashi-2 (MSI2) post-transcriptional processing. MSI2 mediates the posttranscriptional downregulation of HSP90 at the outset of culture and continuously represses the prominent AHR pathway effector CYP1B1 to facilitate haematopoietic stem and progenitor cell (HSPC) expansion. The resulting MSI2-mediated repression of AHR signalling enforces a self-renewal program and allows HSPC expansion ex vivo. Reprinted by permission from Macmillan Publishers Ltd: Nature, Rentas et al, Vol. 532 Pages 508-511, 2016.
Protein-RNA interaction analysis then found that two of the primary RNA targets regulated by Musashi-2 are the 3'UTRs of HSP90 and CYP1B1, both AHR pathway components. The authors thus suggest a model whereby Musashi-2 post-transcriptionally downregulates HSP90 and then continuously represses CYP1B1. The resulting suppression of the AHR signaling pathway, much like SR1 treatment, enforces stem cell self-renewal programming, facilitating ex vivo expansion of human HSCs (Figure).
In Brief
This study adds to a now growing list of recent efforts to expand the numbers of human CD34+ cells for transplantation. Since most umbilical cord blood units in storage today are of insufficient size for adult transplantation, cord blood is often the focus of expansion efforts. However, effective methods of expansion are also likely to be needed beyond cord blood units as gene therapy and gene editing efforts expand indications. The mouse model has been instrumental in hematopoietic research, and in many ways in regards to blood biology, has been a faithful model for clinical translation to humans. In the case of maintaining HSC “stemness” and ex vivo expansion, several of the efforts have been specific to human cells, and do not work in mice, necessitating further exploratory efforts on primary human CD34+ cells as was performed in this recent manuscript.
References
Competing Interests
Dr. Chou and Dr. Hoggatt indicated no relevant conflicts of interest.