Chronic myeloid leukemia (CML) affects approximately one in 100,000 individuals per year and accounts for 15 percent of all new cases of leukemia in the Western Hemisphere. Before the development of targeted therapy with tyrosine kinase inhibitors (TKIs), the median survival was five to seven years.1 CML treatment has changed dramatically over time. Mainstays of medical therapy for CML initially consisted of busulfan or hydroxyurea, followed by interferon-α +/– cytarabine in the 1980s and 1990s; allogeneic transplantation was employed in all phases of the disease.2 Imatinib mesylate, the first TKI to specifically target the BCR-ABL1 oncoprotein,3 was introduced into trials in 1998.4 Imatinib treatment significantly increased the survival and quality of life for patients of all ages, particularly those in chronic phase.5,6 As a result of this and other TKIs, the prevalence of CML continues to rise.7 This, along with the current recommendation to continue TKI therapy on a lifelong basis (several trials are evaluating TKI discontinuation in patients who have achieved a durable complete molecular remission), is likely to greatly impact future health-care costs.
To better quantify the magnitude of the survival gain and its meaning to an individual patient, Dr. Hannah Bower and colleagues determined the life expectancy and the number of expected years of life lost in patients diagnosed with CML in Sweden between 1973 and 2013. This population-based study included 2,662 patients diagnosed with CML in the Swedish Cancer Registry who were monitored until death, censoring, or the end of follow-up. As Swedish law requires that every incidence of cancer is reported to the registry, this study captured the majority of patients with CML in Sweden. Additionally, the study design ensured accurate date of death records. The life expectancy and life-years lost were predicted from a flexible parametric-relative survival model and from results from four selected ages at diagnosis — 55, 65, 75, and 85 years. Only CML patients who were 50 years or older were included in the analysis to optimize the accuracy of the life-years-lost calculation.
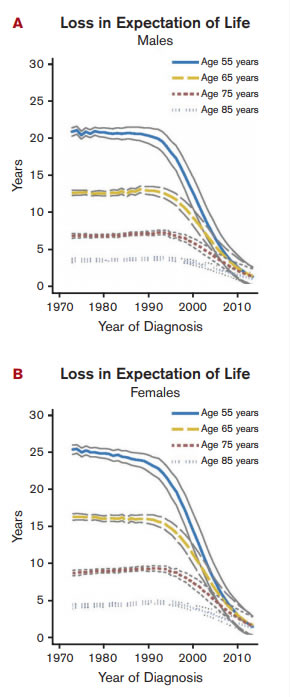
Loss in LEL in Patients With CML by Age and Sex. Loss in expectation of life (LEL) in patients with chronic myeloid leukemia (CML) in Sweden over years of diagnosis by age and sex. LEL is the difference between the life expectancy of the CML population and that in a matched subset of the general population. LEL for all ages at diagnosis in 2010 was less than three years. Reprinted with permission. © 2016 American Society of Clinical Oncology. All rights reserved. Bower, H et al: J Clin Oncol (Life Expectancy of Patients With Chronic Myeloid Leukemia Approaches the Life Expectancy of the General Population) 10.1200/JCO.2015.66.2866.
Loss in LEL in Patients With CML by Age and Sex. Loss in expectation of life (LEL) in patients with chronic myeloid leukemia (CML) in Sweden over years of diagnosis by age and sex. LEL is the difference between the life expectancy of the CML population and that in a matched subset of the general population. LEL for all ages at diagnosis in 2010 was less than three years. Reprinted with permission. © 2016 American Society of Clinical Oncology. All rights reserved. Bower, H et al: J Clin Oncol (Life Expectancy of Patients With Chronic Myeloid Leukemia Approaches the Life Expectancy of the General Population) 10.1200/JCO.2015.66.2866.
The life expectancy of the general Swedish population increased throughout the follow-up period. The life expectancy of the patients with CML of all ages increased dramatically between 1990 and 2013. A diagnosis of CML in 1990 in a 55-year-old woman on average reduced her life expectancy by 24.9 years (95% CI, 24.3-25.6), whereas a diagnosis in 2010 for the same woman reduced her life expectancy by only 2.9 years (95% CI, 1.2 to 4.6). The largest increase in life expectancy, and thus the largest decrease in years of life lost, was seen in younger patients, even though older patients also showed a benefit (Figure). Patients diagnosed in 2010 on average lost less than three years of their life due to their diagnosis, and the life expectancy of patients diagnosed with CML in 2013 approached that of the general population.
The authors suggest that the increased life expectancy data and timing of its development in different age groups reflected in part that imatinib mesylate was approved as second-line CML treatment in Sweden in 2001 and first-line treatment in 2002, and that its use in younger patients predated its use in older patients. They further speculated that as the life expectancy of CML patients of all ages improved prior to 2001, improvements in survival of patients with CML over the years cannot completely be attributable to the introduction of TKIs.
In Brief
A major limitation of this study is that it lacks information on treatments, comorbidities, and cause of death (whether due to progressive disease, cardiovascular events, or secondary malignancies), thus precluding numerous potentially interesting analyses such as the impact of specific variables on life expectancy and life-years lost as a result of CML. A major strength of this study is that it provides population-based information. Additionally, the results are given in patient and provider-centered statistics. From the patient and clinician’s perspective, life expectancy and life-years lost are arguably more meaningful endpoints than many surrogate endpoints commonly employed in CML clinical trials such as complete cytogenetic remission, major molecular remission, or early molecular response.
References
Competing Interests
Dr. Keel indicated no relevant conflicts of interest.