Lifelong production of blood by hematopoietic stem and progenitor cells is regulated by a balance of self-renewal of stem cells, maintaining their limited supply throughout life; and differentiation, allowing for production of all blood lineages. At the 2016 ASH annual meeting, Dr. David Scadden, during his E. Donnall Thomas lecture, described that this coordination of blood production can be due to both inherent traits within hematopoietic stem cells (HSCs) themselves, or due to signals from the surrounding environment or niche. Past research has identified numerous potential epigenetic changes, transcription factor programming, and other stem cell traits that may govern their self-renewal or lineage differentiation choices at an inherent level, as well as stromal cell types, soluble factors, and spatial localization within the bone marrow that may govern stem cell extrinsic regulation of hematopoiesis. Several studies in 2016 have now added evidence that HSC mitochondria may regulate the function of stem cells at both an intrinsic and extrinsic level.
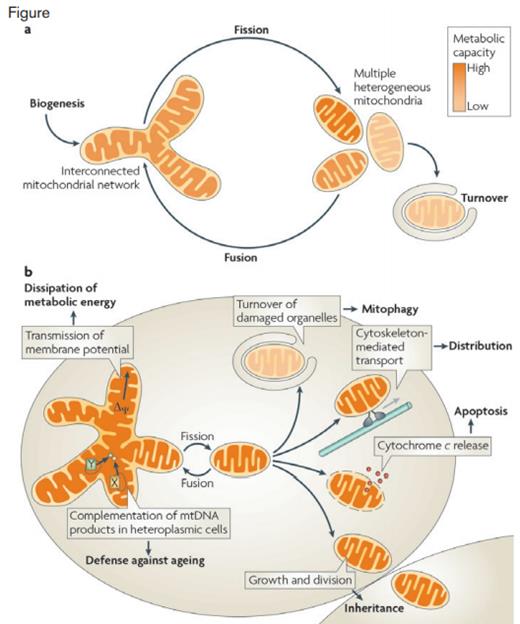
Diagram of the Biological Functions of Mitochondrial Dynamics. A) The mitochondrial life cycle starts with growth and division of pre-existing organelles (biogenesis) and ends with degradation of impaired or surplus organelles by mitophagy (turnover). In between, mitochondria undergo frequent cycles of fusion and fission that allow the cell to generate multiple heterogeneous mitochondria or interconnected mitochondrial networks, depending on the physiological conditions. B) Fusion and fission of mitochondria are important for many biological functions. Division is required for inheritance and partitioning of organelles during cell division, for the release of pro-apoptotic factors from the intermembrane space, for intracellular distribution by cytoskeleton-mediated transport and for turnover of damaged organelles by mitophagy. Fused mitochondrial networks are important for the dissipation of metabolic energy through transmission of membrane potential along mitochondrial filaments and for the complementation of mitochondrial DNA (mtDNA) gene products in heteroplasmic cells to counteract decline of respiratory functions in ageing (X and Y depict alleles of different mitochondrial genes).
Diagram of the Biological Functions of Mitochondrial Dynamics. A) The mitochondrial life cycle starts with growth and division of pre-existing organelles (biogenesis) and ends with degradation of impaired or surplus organelles by mitophagy (turnover). In between, mitochondria undergo frequent cycles of fusion and fission that allow the cell to generate multiple heterogeneous mitochondria or interconnected mitochondrial networks, depending on the physiological conditions. B) Fusion and fission of mitochondria are important for many biological functions. Division is required for inheritance and partitioning of organelles during cell division, for the release of pro-apoptotic factors from the intermembrane space, for intracellular distribution by cytoskeleton-mediated transport and for turnover of damaged organelles by mitophagy. Fused mitochondrial networks are important for the dissipation of metabolic energy through transmission of membrane potential along mitochondrial filaments and for the complementation of mitochondrial DNA (mtDNA) gene products in heteroplasmic cells to counteract decline of respiratory functions in ageing (X and Y depict alleles of different mitochondrial genes).
Prior literature has demonstrated that the transcriptional co-regulator Prdm16 is expressed in HSCs and is important in maintaining their function. While investigating potential mechanisms governing the effect of Prdm16 on hematopoiesis, Dr. Larry Luchsinger and colleagues observed that HSCs without Prdm16 had fragmented mitochondria.1 Normal mitochondria function is maintained by a balance of fission and fusion events, coupled with mitophagy of damaged mitochondria (Figure).2 A shift in the balance toward fission results in an increase in mitochondrial fragments. Along with this hypothesis, the authors found a reduction in the fusion protein mitofusin 2 in Prdm16 knockout HSCs. When they then subsequently knocked out mitofusin 2 in the hematopoietic system and used single HSC transplants in mice, the authors found that strikingly, the mitofusin 2 knockout HSCs exclusively demonstrated a myeloid-biased phenotype, while wild-type HSCs maintained lymphoid potential. There has been increasing appreciation of HSC heterogeneity, with HSCs of differing lineage outputs or potential for engraftment being defined, and the results of this study suggest for the first time that mitochondrial function may specifically regulate a subset of lymphoid HSCs. Given the decline of lymphoid HSCs with age, with a shift toward myeloid-bias production, which phenotypically mimics the mitofusin 2 knockout, the authors suggest that agents targeting mitochondria function may permit therapeutic modulation of lineage fate.
Another publication in 2016 by Dr. Kyoko Ito and colleagues3 identified a subset of HSCs with high expression of the angiopoietin receptor Tie2. Using a Tie2-GFP mouse, the authors demonstrated that this HSC subfraction had an almost sixfold increase in long-term culture-initiating cells (LTC-IC) and a significantly higher ability to reconstitute mice, even in single-cell transplantation assays. After performing single-cell RNA sequencing on Tie2 positive and negative HSCs, a significant enrichment in mitophagy related genes were seen, including Parkin (Park2), Pink1 (PTEN-induced putative kinase 1), Optineurin, Tom 7, Map1lc3a (Lc3), and p62/Sqstm1. The expression of these genes, as well as fatty acid oxidation, were found to be increased after PPAR agonism. When mitophagy was inhibited, by silencing either Park2 or Pink1, HSCs failed to maintain their ability to engraft mice after ex vivo culture, suggesting that under settings of stress, mitophagy may be essential in maintaining stemness during cell division.
These studies highlight that intrinsic regulation of HSCs, whether by directing lineage choice or maintenance of stemness, can be governed by mitochondrial function, and adds an organelle-based mechanism to the ongoing genetic studies exploring the coordinated regulation of hematopoiesis. Intriguingly, a Plenary Scientific Session presentation4 at the 2016 ASH Annual Meeting offered evidence that in addition to fission, fusion, and mitophagy regulation of HSC mitochondria, HSC may actually have the ability to transfer their mitochondria to a neighboring bone marrow stromal cell through cell-to-cell contact reducing their intracellular reactive oxygen species levels and actually stimulating bone formation. This suggests that HSC mitochondria may regulate hematopoiesis in both HSC intrinsic and extrinsic mechanisms, perhaps revealing that HSC mitochondria can bridge the two philosophies discussed by Dr. Scadden during his E. Donnall Thomas lecture.
Surprisingly, a similar cell-to-cell transfer from HSCs to a neighboring stromal cell was previously described,5 demonstrating that intercellular signaling from the HSC to a stromal cell instructed the stromal cell to produce more SDF-1, a positive supporting factor for HSCs. Perhaps, HSCs have the capacity to act as landscapers, altering their surrounding environment to suit their needs. 2017 will certainly shed more light on these HSC-niche interactions.
References
Competing Interests
Dr. Hoggatt indicated no relevant conflicts of interest.