In both children and adults with sickle cell disease (SCD), acute vaso-occlusive pain events (referred to as acute pain) are the most common cause for hospitalization. Acute pain events are devastating to the daily routine of individuals with SCD and their family members, causing disruption in their lives, long wait times in the emergency department and less than optimal pain treatment regimens when admitted to the hospital. The common strategies for inpatient management of acute pain events are nonspecific such as intravenous fluids and opioid therapy. Furthermore, acute pain is a risk factor for life threatening acute chest syndrome and death. For children and adults with SCD, average lengths of inpatient stay for acute pain are approximately four and seven days, respectively. Since the landmark randomized controlled trial demonstrating that hydroxyurea decreases the incidence rate of acute pain,1 many phase II and III clinical trials have been attempted but have failed to definitively demonstrate both decreasing duration and incidence of acute pain events.2
Based on the pathophysiology of acute vaso-occlusive events, Dr. Kenneth I. Ataga and colleagues designed a phase II trial to test the safety and efficacy of a novel therapy — an antibody against the adhesion molecule P-selectin — crizanlizumab. The results suggest a breakthrough in attenuating the high rate of acute pain events in high-risk groups. The double-blind, randomized, placebo-controlled phase II trial assigned participants to receive low-dose crizanlizumab (2.5 mg/kg), high-dose crizanlizumab (5.0 mg/kg), or placebo. The study drug was administered intravenously over 30 minutes, 14 times over the course of one year. The primary endpoint was the annual rate of acute pain episodes and the multiple secondary endpoints include annual rate of days hospitalized, the times to first and second acute pain episodes, and annual rates of other acute vaso-occlusive events including, but not limited to, priapism and acute chest syndrome.
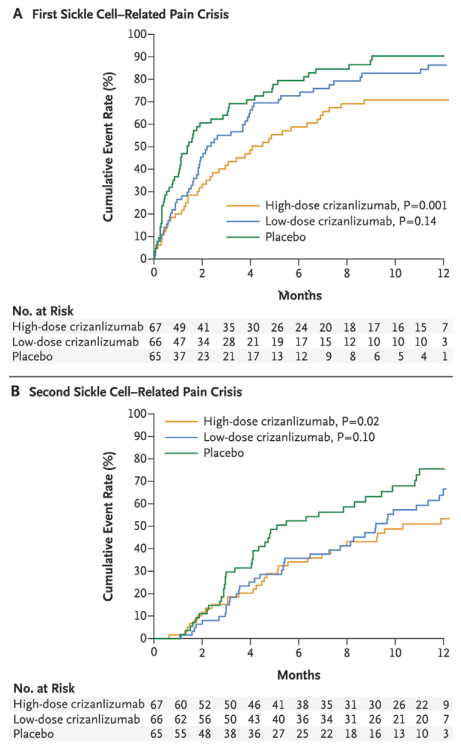
Cumulative Event Rates Among Patients at First (A) and Second (B) Sickle Cell-Related Pain Episodes. From N Engle J Med, Ataga KI et al, Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease, Volume 376, Page 429-39. Copyright © 2017 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Cumulative Event Rates Among Patients at First (A) and Second (B) Sickle Cell-Related Pain Episodes. From N Engle J Med, Ataga KI et al, Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease, Volume 376, Page 429-39. Copyright © 2017 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
The authors of the trial should be congratulated on two accounts: First, in organizing a 60-site double-blind randomized clinical trial in a rare disease — one of the largest trials conducted in SCD; second, in completing the trial in rapid fashion — in less than 18 months. Results of the phase II trial are impressive. In almost every measure, when high-dose crizanlizumab is compared with placebo, statistically significant outcomes favored the active treatment. For the primary outcome measure, there was a 43 percent relative risk reduction in annual acute pain episodes — 1.63 and 2.98 in the high-dose crizanlizumab and placebo treatment groups, respectively (p=0.01). For secondary outcome measures, the median time to the first and second acute pain episode was significantly longer with high-dose crizanlizumab than with placebo (first episode, 4.07 vs. 1.38 months, p=0.001; second episode, 10.32 vs. 5.09 months, p=0.02; respectively). Further, the median time to vaso-occlusive pain events other than acute pain also favored high-dose crizanlizumab over placebo (p=0.02). The rate of adverse events was not significantly different between those receiving active therapy versus placebo. Taken together, these results hold great promise for a new adjunctive therapy for decreasing incidence rates of acute pain episodes in adults with SCD.
Despite the success of the trial, lingering questions remain in conducting a phase II clinical trial in a group of individuals with a rare disease. Two important questions are whether the benefit of the therapy is durable, and is the therapy without serious long-term side effects? The one-year trial simply cannot address either question. Other unanswered questions refer to costs and how to address the system delivery challenges of giving an intravenous therapy monthly for a prolonged period to a large number of individuals with SCD. One could imagine infusions centers where the therapy could be given efficiently, but the challenges of monthly intravenous therapy in a large adult population of individuals with SCD are considerable.
In Brief
In conclusion, since the U.S. Food and Drug Administration (FDA) approval of hydroxyurea therapy in 1998, the completion of the crizanlizumab phase II trial has potential for now a second FDA-approved drug for the prevention of acute pain episodes in SCD. However, more definitive answers are required before health care providers can confidently explain both short and long term benefits as well as risks of this novel therapy to individuals with SCD and their family members.
References
Competing Interests
Dr. DeBaun indicated no relevant conflicts of interest.