In 1955, Dr. Emil J. Freireich had the privilege of serving in the U.S. Public Health Service as a commissioned officer when he was drafted into the military. Freireich then obtained a position at the National Cancer Institute (NCI) in the new Clinical Center on the campus of the National Institutes of Health. Dr. Gordon Zubrod had been recently recruited from St. Louis University as clinical director of the NCI. He in turn recruited his chief resident, Dr. Emil Frei III, to become the head of chemotherapy research. When Dr. Freireich was interviewed by Dr. Zubrod, he informed him that he had completed two years of a hematology fellowship at Boston University, had made important new observations, and had few publications of note. Dr. Zubrod noted that although he had no previous training in pediatrics, because of his excellent hematology training he would be assigned to care for children with acute lymphoblastic leukemia (ALL).
Dr. James Holland from Columbia University preceded Dr. Freireich by approximately one year. He had begun to attract referrals of children with ALL to study treatment with the three then-known active agents — methotrexate, 6-mercaptopurine, and prednisone — each of which could induce a small percentage of complete remissions that lasted for short durations of about eight weeks. Back then, 100 percent of children with ALL died within a year. When Dr. Holland left for Roswell Park Memorial Institute in Buffalo, NY, to head their oncology program, Dr. Freireich was assigned to take care of the children. When he began his tenure, the academic medical community was very skeptical of treatment with these drugs and felt that they just prolonged the suffering of children with leukemia. Dr. Zubrod served during World War II in the malaria project, which used the modern sophisticated techniques of randomized controlled clinical trials, preclinical toxicology, and animal models. During his clinical years as an infectious disease expert, he had learned that when there is more than one agent active against a disease, specifically tuberculosis, the combination of two agents offered better results than the sequence. He therefore suggested to Drs. Frei and Freireich that they study the combination of the two most active agents, methotrexate and 6-mercaptopurine, and compare the results to single agents.
Protocol One began under the chairmanship of Dr. Frei. Dr. Holland, now in Buffalo, agreed to join the study, thus creating the first cooperative leukemia chemotherapy group in the world. Protocol One was a combination of 6-mercaptopurine and methotrexate, comparing two schedules of methotrexate, one dose every four days and daily, because methotrexate had been shown in animal models to be more beneficial if given intermittently (based on work done by Dr. Alexander Goldin). To combine the two drugs, it was necessary to know the maximum tolerated dose (MTD). Pharmacology studies were done in animals by Dr. David Rall who demonstrated that a dose of 60 percent of the MTD of each of the two drugs would equal the MTD of a combination. This principle is still followed today—combining agents of similar toxicities at two-thirds of the single-agent doses. They recruited approximately 60 patients and showed that the combination resulted in higher complete response rates and longer survival durations than either agent used alone. However, the efficacy of the two methotrexate schedules did not differ. When these results were published, 10 additional academic institutions volunteered to join the cooperative group, which allowed Protocol Two to accrue data significantly faster. Protocol Two compared the combination of 6-mercaptopurine and methotrexate to the sequence of the two drugs (methotrexate followed by 6-mercaptopurine and 6-mercaptopurine followed by methotrexate). Dr. Lloyd Law, who developed the L-1210 leukemia model in mice, had shown that when the transplanted tumor L-1210 became resistant to one of the two agents, the sequential use of the second agent showed enhanced effectiveness — so-called “collateral sensitivity.” They had rapid accrual, enrolling more than 100 patients in a short time, and the results showed that the simultaneous combination was superior to either sequence, without evidence of collateral sensitivity.
Shortly after Protocol Two, they conducted studies combining prednisone with each of the myelosuppressive drugs since prednisone did not produce cytopenias. They showed that the combinations were always more effective. Dr. Irving Johnson of Eli Lilly had discovered an alkaloid from the periwinkle plant that was highly active against a form of transplantable leukemia, but not active against L-1210 leukemia. He asked if they would be interested in conducting a phase II study (they had already established the MTD in phase I). Thus began a study with this single agent, vincristine, in children refractory to methotrexate, 6-mercaptopurine, and prednisone. The results, published by Dr. Myron Karon, were dramatic: More than two-thirds of children with advanced ALL achieved complete remission with this single agent. Remissions were short, but there was no myelosuppression. This led to the concept of combining vincristine, methotrexate (amethopterin), 6-mercaptopurine, and prednisone into a four-drug regimen that they called “VAMP.” The results of the VAMP program in the first 30 children were exceptional. Greater than 90 percent of children with ALL achieved complete remission and the remissions were somewhat longer (about 3 months). However, Dr. Freireich and colleagues realized that treatment with two to three courses was not sufficient; they then designed a regimen called “early intensification,” which used the same drugs in remission at doses that achieved complete remission. After three courses of early intensification, therapy was discontinued, and while remission durations were substantially prolonged, relapses occurred in all children. The investigators then designed a regimen called “intermittent re-induction,” where VAMP was continued at regular intervals for a year following early intensification. At the end of that year, 30 percent of children remained in remission, an event that had never occurred before. This produced the first regimen ever that cured a fraction of children with ALL.
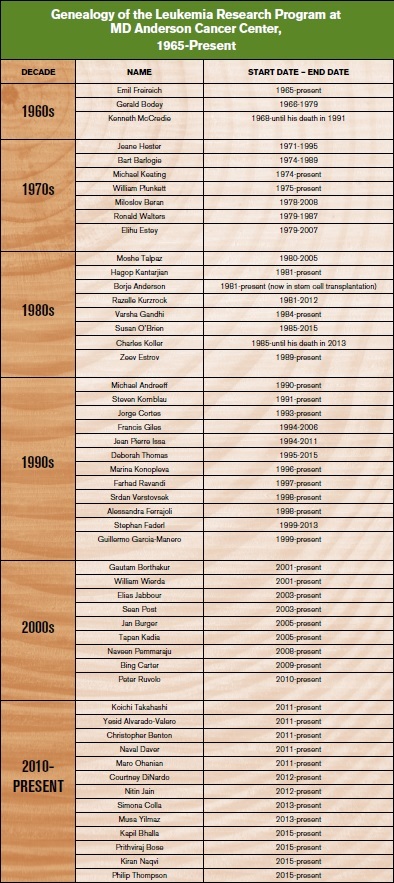
In 1946, Dr. R. Lee Clark was named as the first permanent director of a new cancer institute, MD Anderson Hospital and Tumor Institute. Dr. Clark was a surgeon and had participated in radical surgery attempting to remove both local and regional disease. Still, recurrences occurred in the regions of massive surgery. Dr. Clark recruited Dr. Gilbert Fletcher, a radiation therapist from France who came to MD Anderson and established the first cobalt-generated high-energy radiation therapy. His use of irradiation to the tumor region in the postoperative adjuvant setting demonstrated good local control. However, systemic relapses still occurred. Dr. Clark, an imaginative and creative physician scientist, observed the events at MD Anderson and decided to add chemotherapy to control cancer. He recruited Dr. Frei in early 1965, who in turn recruited Dr. Freireich. They investigated the four-drug combination therapy in acute leukemia in adults. Thus was created the first leukemia program in the world, focused on adult leukemia research and therapy.
In the late 1960s, very little was known about cancer research and therapy, and the idea of tumor-specific programs had not been conceived. Dr. Freireich not only initiated the adult leukemia program, he also recruited numerous visionary experts who worked within a newly created Department of Developmental Therapeutics. From 1966 until 1979, “Developmental Therapeutics” expanded at MD Anderson to grow into the largest cancer program in the world, becoming home to more than 100 cancer experts who specialized in different tumors. These included some of the then most famous researchers in the field: Drs. Jordan Gutterman, Evan Herch, Michael Keating, Fernando Cabanillas, Gabriel Hortobagyi, George Blumenschein, Bart Barlogie, Gerald Bodey, Robert Benjamin, Sewa Legha, and many others. Among them was Dr. Kenneth B. McCredie who heard Dr. Freireich give a talk about curing ALL in Australia. He was so inspired that he packed his family and showed up one night at the door of Dr. Freireich’s home, asking for a job. He had the dream and vision to cure leukemia. Dr. McCredie was hired as the first leader of the adult leukemia program from 1969 until 1980. Dr. Keating joined MD Anderson as a fellow in 1974, and Dr. Elihu Estey joined in 1978. They became the third and fourth long-term faculty in the Leukemia Section. In 1979, Dr. Charles LeMaistre became the second MD Anderson Cancer Center president with the vision of creating tumor-specific programs. This resulted in the creation of the Division of Cancer Medicine within the Department of Hematologic Malignancies: Dr. Freireich led the Department, and Dr. McCredie ran the Leukemia Section.
Dr. Hagop Kantarjian visited MD Anderson as a medical student for four months in 1978, joined MD Anderson as a fellow in 1981 and was recruited to be part of the leukemia faculty in 1983. In rapid succession, the Leukemia Section recruited several visionary leukemia specialists who were infected with the dream of curing leukemia. Most of them remained as leukemia experts in the subsequent Department for most of their careers. These included Drs. Ronald Walters, Susan O’Brien, Michael Andreeff, Miloslav Beran, Elihu Estey, William Plunkett, Zeev Estrov, Borje Anderson, Moshe Talpaz, Razelle Kurzrock, Alessandra Ferrajoli, Varsha Gandhi, Jorge Cortes, Guillermo Garcia-Manero, William Wierda, Srdan Verstovsek, Gautam Borthakur, Elias Jabbour, Marina Konopleva, Francis Giles, Farhad Ravandi, and numerous others. The Leukemia Section later expanded into the Leukemia Department in 1994, under the Division of Cancer Medicine. The Leukemia Department started with seven faculty and 10 leukemia research staff. In 2016, the Department was home to 40 leukemia faculty, 80 mid-levels, 10 PharmDs, and 300 research staff. In 2016, more than 2,000 new patients with leukemia (including myelodysplastic syndromes [MDS] and myeloproliferative neoplasms [MPN]) were referred to MD Anderson, and the leukemia faculty oversaw an inpatient service of 120 beds. The Leukemia Department would continue its mission of research-focused leukemia discoveries for the next three to four decades.
The leukemia research program created multiple research initiatives that resulted in important discoveries, many of which became standards of care and improved survival in leukemia subsets. Prominent among these discoveries are the following:
Hairy cell leukemia: The discovery of the activity of interferon. Later confirmatory studies of the efficacy of cladribine, and the discovery of the efficacy of rituximab. This led to the development of the cladribine + rituximab regimen, associated with a 10-year disease-free survival of 80 percent.
Acute myeloid leukemia: The development of cytarabine, cytarabine + anthracycline combinations, and high-dose cytarabine regimens in 1983. The expansion of the discovery of the activity of ATRA and arsenic trioxide in acute promyelocytic leukemia, and the development of the ATRA + arsenic trioxide regimen. The development of nucleoside combinations with cytarabine and antracyclines (e.g., FLAG IDA). The development of epigenetic therapy with decitabine.
Chronic myeloid leukemia (CML): The creation of the definitions of accelerated phase and of cytogenetic response criteria. The discovery of the activity of interferon. Later numerous developmental programs with tyrosine kinase inhibitors (TKIs) including imatinib, high-dose imatinib, dasatinib, nilotinib, bosutinib, and ponatinib. These endeavors in long-term historical studies demonstrated the survival benefit with TKIs. The development of omacetaxine for the treatment of CML.
ALL: The development of the hyper-CVAD regimen in 1992. Subsequent follow-up regimens including hyper-CVAD plus TKIs in Ph+ ALL, hyper-CVAD + rituximab in Burkitt Leukemia and pre-B-ALL. The development of clofarabine and liposomal vincristine. Replacement of CNS radiation prophylaxis with intrathecal chemotherapy prophylaxis. The discovery of the activity of inotuzumab ozogamycin in 2010. The US development of blinatumomab and other monocloncal antibodies as single agents and in combinations in ALL. These studies have established new standards of care and have improved long-term survival in adult ALL. Several of the discoveries were then incorporated also into the pediatric regimens (CNS intrathecal prophylaxis; clofarabine; combinations of TKIs and chemotherapy in Ph+ ALL; monoclonal antibodies).
MDS and MPNs: The development of decitabine in MDS. The development of ruxolitinib and investigation of multiple JAK2 inhibitors in myelofibrosis.
Chronic lymphocytic leukemia: The discovery of the activity of fludarabine (F). Later development of F + cyclophosphamide (FC), and FC + rituximab (FCR; Keating regimen). Original studies with the new B-cell receptor inhibitors including ibrutinib, idelalisib, and with the Bcl-2 inhibitor venetoclax.