The nucleoside analog cytarabine (ara-C) is heavily utilized in the treatment of patients with newly diagnosed and relapsed acute myeloid leukemia (AML). Unfortunately, the outcome of AML in adults is poor, and lack of response to ara-C is a common cause for treatment failure. Despite the use of ara-C for more than three decades, two recent studies have now identified that an enzyme present in AML cells, termed SAMHD1 (SAM domain and HD domain 1), depletes the active metabolite of ara-C. This is an exciting discovery because SAMHD1 inhibition promotes the cytotoxicity of ara-C, and the level of SAMHD1 expression seems to predict the response of AML patients to ara-C–based treatment regimens.
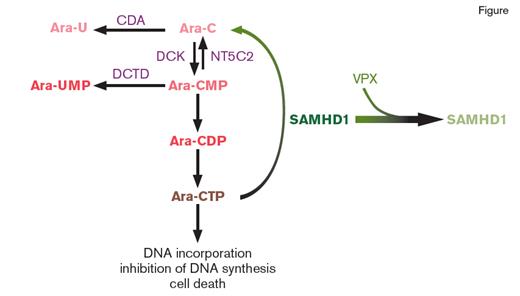
Diagram of the Role of SAMHD1 in Cytarabine (ara-C) Metabolism. Upon entering cells, ara-C is converted into its active metabolite ara-C triphosphate (ara-CTP). Ara-CTP, in turn, results in lethal misincorporation into genomic DNA. The enzyme SAMHD1 limits ara-C cytotoxicity by hydrolyzing ara-CTP. Interestingly, an accessory protein, termed viral protein X (VPX), encoded by several viruses recruits SAMHD1 to a cullin4A-RING E3 ubiquitin ligase, targeting SAMHD1 for proteasomal degradation. Depletion of SAMHD1 in this method promotes sensitivity of cells to ara-CTP. The expression level of other proteins responsible for ara-C transport metabolism as shown in this figure do not correlate with response to ara-C mediated cell death. Abbreviations: cytidine deaminase (CDA), deoxycytidylate deaminase (DCTD), deoxycytidine kinase (DCK), 5'-nucleotidase-II (NT5C2).
Diagram of the Role of SAMHD1 in Cytarabine (ara-C) Metabolism. Upon entering cells, ara-C is converted into its active metabolite ara-C triphosphate (ara-CTP). Ara-CTP, in turn, results in lethal misincorporation into genomic DNA. The enzyme SAMHD1 limits ara-C cytotoxicity by hydrolyzing ara-CTP. Interestingly, an accessory protein, termed viral protein X (VPX), encoded by several viruses recruits SAMHD1 to a cullin4A-RING E3 ubiquitin ligase, targeting SAMHD1 for proteasomal degradation. Depletion of SAMHD1 in this method promotes sensitivity of cells to ara-CTP. The expression level of other proteins responsible for ara-C transport metabolism as shown in this figure do not correlate with response to ara-C mediated cell death. Abbreviations: cytidine deaminase (CDA), deoxycytidylate deaminase (DCTD), deoxycytidine kinase (DCK), 5'-nucleotidase-II (NT5C2).
Ara-C results in cell death by being converted into its active metabolite, ara-C triphosphate (ara-CTP), which causes DNA damage by perturbing DNA synthesis. SAMHD1 was previously discovered to serve as a dNTP (deoxynucleotide) triphosphohydrolase in studies that identified SAMHD1 as an enzyme that reduces HIV-1 infectivity in cells by limiting dNTPs available for reverse transcription. Now, both Dr. Nikolas Herold and colleagues, as well as Dr. Constanze Schneider and colleagues, have identified that ara-CTP is also a direct substrate of SAMHD1 (Figure). Consistent with this finding, depletion of SAMHD1 expression by RNA interference or CRISPR-Cas9–mediated disruption markedly sensitizes cells to ara-C toxicity. Conversely, overexpression of SAMHD1, but not a dNTPase-deficient SAMHD1 mutant, promotes resistance to Ara-C. Moreover, AML cell lines with acquired resistance to ara-C have increased expression of SAMHD1, coincident with dramatically increased ara-CTP levels.
Given these data, both groups evaluated whether expression of SAMHD1 at the mRNA or protein level might correlate with response to ara-C in AML patients. In AML cell lines, SAMHD1 expression inversely correlated with ara-C sensitivity. Interestingly, no such similar correlation was seen with other proteins essential in ara-C uptake into cells (such as SLC29A1) or in its conversion to ara-CTP (DCK, CDA, DCTD, NT5C2; Figure). Finally, levels of SAMHD1 mRNA expression correlated with clinical response to ara-C–based therapy in multiple distinct cohorts of pediatric and adult AML patients. These data therefore nominate SAMHD1 as a potentially important and reliable biomarker for predicting response of individual AML patients to ara-C–based therapy.
Importantly, from a drug development perspective, targeting SAMHD1 for proteasomal degradation also improved sensitivity to Ara-C. This was achieved through the use of virus-like particles (VLPs) that shuttle the SAMHD1 interacting lentiviral Vpx protein (Vpx-VLPs) into cells, thereby recruiting SAMHD1 to a cullin4A-RING E3 ubiquitin ligase for degradation (Figure). Again here, use of Vpx results in increased cellular Ara-CTP levels.
In Brief
Given the requirement of dNTPs for the efficacy of nucleoside analogs other than ara-C, the authors of both studies evaluated the effects of SAMHD1 depletion on the response to multiple chemotherapeutic agents. In these experiments, SAMHD1 depletion also promoted sensitivity to the purine analog fludarabine and clofarbine, but not to the unrelated cytotoxic compounds etoposide or daunorubicin. These data suggest that SAMHD1 should be evaluated in response to all nucleoside-analog–based drugs in future studies. Based on these results, further efforts to develop drugs to inhibit SAMHD1’s enzymatic activity and evaluate the clinical utility of SAMH1-degrading Vpx-VLPs may result in a means to increase the efficacy of Ara-C–based treatment in AML.
Competing Interests
Dr. Abdel-Wahab indicated no relevant conflicts of interest.