Until recently, two major barriers limited cure for individuals with sickle cell disease (SCD). First, for both children and adults, there has been a paucity of donors — estimated at only 18 percent of those considering hematopoietic stem cell transplantation (HSCT).1 Second, myeloablative conditioning regimens had typically been too toxic for adults. Over the past 10 years, however, two strategies have emerged to address these intrinsic challenges, making cure a realistic outcome in an increasing number of children and adults with SCD. The first strategy is nonmyeloablative HSCT; it recently has been successfully applied in adults with SCD, with HLA-matched sibling donors. To increase the pool of donors, the most promising experimental strategy is the use of haploidentical transplantation with posttransplant cyclophosphamide — a nonmyeloablative strategy2 with greater than 90 percent donor availability. Gene therapy is now a second strategy to increase the donor pool, at least in children with SCD.
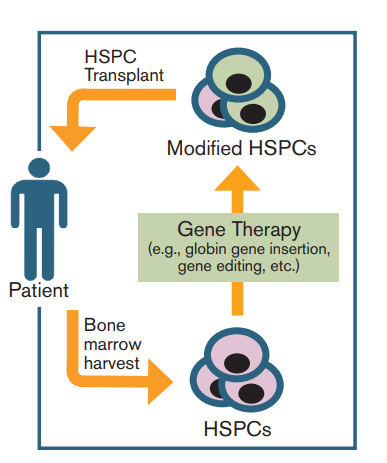
Diagram of the Typical Process of Gene Therapy for Hematopoietic Disorders. Hematopoietic stem and progenitor cells (HSPCs) can be modified directly, as is the case for currently used therapies. The genetically modified HSPCs are then transplanted back into the patient. When HSPCs are modified directly, modification may not occur in every cell. Goodman MA et al, Ther Adv Hematol. 15(5):302-315, copyright © 2016 by SAGE Publications. Reprinted by Permission of SAGE.
Diagram of the Typical Process of Gene Therapy for Hematopoietic Disorders. Hematopoietic stem and progenitor cells (HSPCs) can be modified directly, as is the case for currently used therapies. The genetically modified HSPCs are then transplanted back into the patient. When HSPCs are modified directly, modification may not occur in every cell. Goodman MA et al, Ther Adv Hematol. 15(5):302-315, copyright © 2016 by SAGE Publications. Reprinted by Permission of SAGE.
Dr. Jean-Antoine Ribeil and colleagues should be congratulated on performing the first ever successful gene therapy trial in SCD. The technical, scientific, and research governance barriers were significant, and the authors clearly addressed each one successfully. Equally laudable is the bravery of the participant and the participant’s family. The family’s altruism and trust in both their clinical and research teams should not be taken lightly. The gene therapy was designed using the LentiGlobin BB305 (Bluebird Bio) vector, which encodes for the human hemoglobin B genetic variant. Briefly, bone marrow harvest was obtained twice, and CD34+ (stem) cells were transduced with the LentiGlobin BB305 vector (Figure). Next, a myeloablative dose of busulfan with area under the curve 19,363 μmol/min was administered, and after a two-day washout period, the transduced CD34+ cells (5.6 × 106 CD34+ cells/kg) were infused. Fifteen months after completion of the procedure, the participant did not report any vaso-occlusive pain episodes, and the level of donor beta globin production was approximately 50 percent.
Why the optimism? As a proof of principle, gene therapy for SCD is a therapeutic paradigm shift. Theoretically, donor availability is no longer an obstacle for children and adults with SCD. With the rapid pace of transplant biology research, conditioning regimens are expected to evolve from myeloablative to nonmyeloablative approaches. Until such advances, gene therapy will most likely be restricted to children rather than adults with SCD who may not tolerate the current high dose of busulfan.
Why the caution? Children with SCD living in high-income countries no longer have a life-threatening disease, but rather a chronic disease with disease-associated, life-threatening events. Two large observational studies in children with SCD indicated 15-year–3 and 16-year4 Kaplan-Meier survival estimates of approximately 99 percent with and without hydroxyurea therapy, respectively. A third cohort study of children with SCD from a region in Paris, France, indicated that after introduction of the online guidelines, there was also an increase in the overall five-year survival from 98.3 percent to 99.2 percent.5 In terms of morbidity, the rate of stroke in a population of children with SCD can drop a log-fold at SCD centers screening with transcranial Doppler and subsequently treating those with abnormal values with regular blood transfusions for at least a year, then switching to therapy with hydroxyurea indefinitely. For children with SCD living in low- and middle-income countries, where approximately 90 percent of all children with SCD are born, gene therapy is not an option. In summary, gene therapy to treat children with SCD is currently restricted to those who: 1) have severe disease; 2) have failed blood transfusion or hydroxyurea therapy; 3) require treatment for comorbidities; 5) live in a high-income country; and 6) are not likely to die from the disease in childhood. Given the known short-and long-term toxicities of myeloablative doses of busulfan, the calculated trade-off of gene therapy is that the benefits will outweigh the unknown late potential adverse effects of busulfan in this population.
In Brief
The bottom line is that gene therapy provides a new option for curing SCD, a disease that still remains with only one Food and Drug Administration–approved disease-modifying agent, hydroxyurea. The SCD community remains cautiously optimistic that some of the intrinsic challenges with gene therapy for SCD will be overcome with the rapid advancement of transplant immunobiology.
References
Competing Interests
Dr. DeBaun indicated no relevant conflicts of interest.