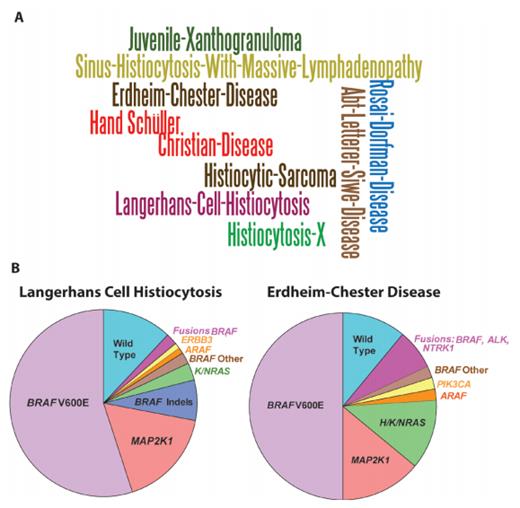
Toward a Molecular Genetic Understanding of Histiocytic Neoplasms A) Word cloud of the various names and eponyms for systemic histiocytic neoplasms that have been used since the initial descriptions of the diseases 150 years ago. B) Pie charts of somatic mutations affecting MAP kinase signaling that are now known to occur in Langherans cell histiocytosis and Erdheim-Chester disease. The majority of recurrent mutations that have been identified are mutually exclusive activating mutations affecting MAP kinase signaling, with the most common being the BRAF V600E mutation. Reprinted with permission from the American Association for Cancer Research.
Toward a Molecular Genetic Understanding of Histiocytic Neoplasms A) Word cloud of the various names and eponyms for systemic histiocytic neoplasms that have been used since the initial descriptions of the diseases 150 years ago. B) Pie charts of somatic mutations affecting MAP kinase signaling that are now known to occur in Langherans cell histiocytosis and Erdheim-Chester disease. The majority of recurrent mutations that have been identified are mutually exclusive activating mutations affecting MAP kinase signaling, with the most common being the BRAF V600E mutation. Reprinted with permission from the American Association for Cancer Research.
Histiocytic neoplasms (or histiocytoses) describe a group of diseases believed to be derived from dendritic cell, monocyte, and/or macrophage lineages, which result in an accumulation of lesional cells and ensuing damage in a variety of tissues throughout the body. The protean clinical manifestations of histiocytoses, which affect children as well as adults, combined with their diverse histologic presentation and rarity, has made these diseases among the most challenging hematologic disorders to diagnose and categorize. In fact, for decades, histiocytoses such as Langerhans cell histiocytosis (LCH), Erdheim-Chester disease (ECD), and juvenile xanthogranuloma (JXG) were considered to be inflammatory, non-neoplastic conditions, with potentially similar origins to hemophagocytic lymphohistiocytosis (HLH). However, a series of discoveries regarding the molecular genetic causes of histiocytoses over the last seven years has reshaped our understanding of nearly all subtypes and has led to potent targeted treatments for patients affected by these conditions (Figure 1). We now understand that LCH, ECD, and JXG are clonal disorders with a high frequency of somatic mutations resulting in activation of the MAP kinase signaling pathway. These advances have been described in a number of excellent recent reviews.1,2 In this article, we summarize some of the most important findings regarding histiocytoses.
Classification of Histiocytoses
In the World Health Organization (WHO) classification of hematopoietic malignancies, histiocytic neoplasms are included under the rubric of “mature lymphoid, histiocytic, and dendritic neoplasms.”3 There are currently nine WHO-recognized entities including histiocytic sarcoma, LCH, Langerhans cell sarcoma, indeterminate dendritic cell tumor, interdigitating dendritic cell sarcoma, follicular dendritic cell sarcoma, fibroblastic reticular cell tumor, disseminated JXG, and ECD. These are currently differentiated from one another based on histologic and/or immunophenotypic characteristics with distinct genetic alterations only defined for a few. Additionally, it is also important to be aware of an alternate classification system for histiocytic and dendritic cell neoplasms recently proposed by the Histiocyte Society.4 The different eponyms that has been used for systemic histiocytoses are shown in Figure 1A.
Somatic Mutations Drive Histiocytoses
Despite categorization of histiocytoses with lymphoid neoplasms in the WHO classification, it is important to note that 1) gene expression analyses of LCH and ECD indicate that these disorders bear greater resemblance to myeloid lineage cells than dendritic cells5,6 ; 2) genetic analyses have identified that mutations in histiocytosis lesional cells can be found in CD34+ and circulating myeloid cells in patients6,7 ; and 3) functional analyses suggest that at least some histiocytoses are derived from hematopoietic precursors.8 These observations suggest that LCH, ECD, and JXG may actually be more appropriately considered clonal disorders of the myeloid lineage.
Interestingly, a series of studies performing mutational analysis of histiocytosis lesional biopsies has identified that both LCH and ECD are characterized by approximately 50 percent of patients having a BRAF V600E mutation (Figure 1B). The BRAF V600E mutation is common to a variety of epithelial cancers, strongly promotes activation of the MAP kinase pathway, and sensitizes cells to inhibitors of this pathway. Further studies to define mutations present in BRAF-wildtype patients have since identified that nearly all LCH and ECD patients have a mutation activating the same pathway as BRAF V600E mutations. This includes mutations in MAP2K1 (encoding the MEK1 kinase just downstream of BRAF), NRAS, KRAS, and ARAF as well as activating translocations in BRAF, ALK, and NTRK1 (reviewed recently9 ). The high frequency of mutations in MAP2K1 and ARAF make LCH and ECD quite unusual in that these kinases are much less frequently mutated in any other form of cancer. In addition to the above, genetic analysis of indeterminate dendritic cell histiocytosis identified that a high frequency of these tumors have a specific translocation (ETV3-NCOA2) that appears to define this histologic entity.10
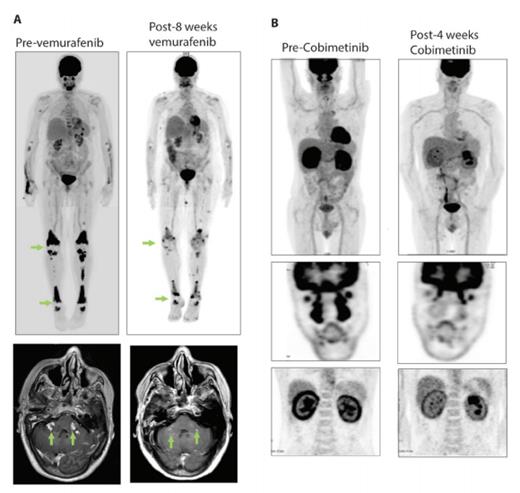
Examples of Responses of BRAF V600E and MAP2K1 Mutant Adults with Erdheim-Chester Disease (ECD) to Molecularly Targeted Therapies. A) Positron emission tomography (PET) scan and brain MRI of a BRAF V600E-mutant ECD patient with skeletal and parenchymal brain lesions pre- and eight-weeks post-treatment with the BRAF inhibitor vemurafenib. B) PET scan of a MAP2K1 Q56P-mutant ECD patient with disease infiltration in facial sinuses, heart, and kidneys pre- and four-weeks post-treatment with the MEK 1/2 inhibitor cobimetinib.
Examples of Responses of BRAF V600E and MAP2K1 Mutant Adults with Erdheim-Chester Disease (ECD) to Molecularly Targeted Therapies. A) Positron emission tomography (PET) scan and brain MRI of a BRAF V600E-mutant ECD patient with skeletal and parenchymal brain lesions pre- and eight-weeks post-treatment with the BRAF inhibitor vemurafenib. B) PET scan of a MAP2K1 Q56P-mutant ECD patient with disease infiltration in facial sinuses, heart, and kidneys pre- and four-weeks post-treatment with the MEK 1/2 inhibitor cobimetinib.
Therapeutic Targeting of BRAF and MEK in Histiocytosis
Based on the success of targeting BRAF V600E mutant melanoma with RAF and MEK inhibitors, the discovery of BRAF V600E-mutant LCH and ECD led to efforts to determine the efficacy of these agents for adults with histiocytosis. A number of clinical studies have now demonstrated the efficacy of vemurafenib for BRAF V600E-mutant histiocytosis. In the one clinical trial that has been published, a cohort of 22 ECD and four LCH patients experienced a response rate of 64 percent to vemurafenib (Figure 2A).11 Extended follow-up of this study has identified that these responses are durable, with a median treatment duration now of 14.9 months (range, 2-43 months).12
Given that the use of vemurafenib requires documentation of the BRAF V600E mutation combined with the fact that nearly 50 percent of histiocytosis patients lack this mutation, there has been an ongoing effort to identify targeted therapies for BRAF-wildtype patients. Currently, it appears that the use of single-agent MEK inhibitors, including trametinib or cobimetinib, may have great efficacy for adults with BRAF-wildtype LCH or ECD (Figure 2B). Despite promising initial data on this approach, it is important to realize that it is not yet known if the variety of activating MEK1 mutations all respond to MEK1/2 inhibition nor is it understood which MEK inhibitor is ideal for use in histiocytosis. In order to determine the safety and efficacy of single-agent MEK inhibition based on the diverse genetic alterations present in BRAF-wild-type histiocytosis patients, our group has an ongoing phase II trial of single-agent cobimetinib for adults with histiocytic disorders (ClinicalTrials.gov identifier NCT02649972).
Conclusions and Unanswered Questions
The discovery of recurrent clonal mutations activating the MAP kinase pathway in histiocytosis as well as the response of these patients to small molecules inhibiting this pathway has been remarkable. Despite these advances, there is a need to continue genetic analysis of the variety of histologically defined forms of histiocytosis other than LCH, ECD, and ICH to determine if these conditions also harbor high frequencies of somatic mutations, some of which may be important in molecular diagnosis or treatment. Additionally, there is a need to more conclusively define the cellular origin(s) of LCH and ECD. Although accumulating data suggest that these conditions are derived from hematopoietic progenitors and/or myeloid progenitors, it also is possible that more than one cell of origin may characterize these conditions.
References
Competing Interests
Dr. Abdel-Wahab indicated no relevant conflicts of interest.