The Case
A 14-year-old girl was diagnosed with acute lymphoblastic leukemia (ALL) and underwent therapy with a standard ALL regimen. Unfortunately, the disease relapsed within 10 months following completion of treatment and an allogeneic stem cell transplant was performed from a sibling donor. Relapse of ALL occurred again after seven months and at this stage the patient was considered for therapy in a clinical trial of CD19-specific chimeric antigen receptor (CAR-) T cell therapy.
The Question
What are the practical issues that hematologists need to know about during the introduction of CAR-T cell therapy?
The Response
CAR-T cell therapy promises to rewrite the lexicon of cancer treatment. Current therapies have made remarkable advances in first-line management of conditions such as ALL and diffuse large B-cell lymphoma (DLBCL). Survival curves have improved almost inexorably, particularly for younger patients, but huge challenges remain. A substantial proportion of patients fail to respond to first-line therapy, treatment is prolonged and intensive, and in the setting of disease relapse, the options become depressingly limited. Is it too much to believe that CAR-T cells will address all of these challenges?
B-cell ALL will see the first widespread application of CAR-T cells. Indeed on July 12, 2017, Novartis announced that the 10 members of the Oncologic Drugs Advisory Committee of the U.S. Food and Drug Administration (FDA) had voted unanimously to recommend approval of tisagenlecleucel (CTL019) for the treatment of relapsed or refractory pediatric and young adult patients with ALL. This positive assessment was based largely on the ELIANA study (NCT02435849), which was the first pediatric global CAR-T cell therapy registration trial and which took place at 25 centers in the United States, Canada, European Union, Australia, and Japan.
CAR-T cell therapy has been developed from relatively small clinical studies within academic centers, and very few hematologists have experienced the practical challenges of managing a CAR-T cell protocol. This profile will now change at a considerable pace, and cellular therapy is likely to become a “standard option” for patients across many countries.
The selection of patients for CAR-T cell therapy will be limited by future license, cost, and access to clinical studies. The approach is likely to become a standard option for patients with relapsed/refractory ALL.1,2 Responses in patients with relapsed DLBCL are so encouraging that this indication is likely to follow in short order. Response rates in chronic lymphocytic leukemia are less certain, but even in this most common of conditions, options are badly needed for patients who relapse from current oral therapies. Indeed, CD19 is present on most B cell malignancies, and as such, mantle cell lymphoma, marginal zone lymphoma, follicular lymphoma, and Waldenström macroglobulinemia are also in play.
Methodology
Collection and transduction of autologous T cells is achieved by leukapheresis, from which cells such as monocytes and B cells are removed before the T cells are activated with mitogens such as anti-CD3/CD28 antibody-coated beads. Lentiviral or retroviral vectors carrying the CAR construct are then used to transduce activated T cells over one to two days before they are expanded in the laboratory and cryopreserved. Currently this complex process is restricted to a few specialized manufacturing sites. As the applications of CAR-T cells expand, it seems reasonable to consider that this may be extended to include additional sites, perhaps even to local units that undertake processing of stem cell collections. Transduced cells can then be shipped to the clinical site following release testing.
Table. Comparison of peripheral blood transgene cellular kinetic parameters in responders vs. non-responders - pooled data from Studies B2202 and B2205J
| Parameter . | Statistics . | ResponderN=62 . | Non-responderN=8 . |
|---|---|---|---|
| AUC0-28d(copies/μg DNA x days) | n | 61 | 6 |
| Geo-mean (CV%) | 318,000 (177.8) | 156,000 (99.4) | |
| Cmax | n | 61 | 7 |
| Geo-mean (CV%) | 55,700 (155.4) | 20,000 (71.6) | |
| Tlast | n | 62 | 8 |
| Median (min, max) | 102 (17.8, 380) | 27.8 (20.9, 83.9) |
| Parameter . | Statistics . | ResponderN=62 . | Non-responderN=8 . |
|---|---|---|---|
| AUC0-28d(copies/μg DNA x days) | n | 61 | 6 |
| Geo-mean (CV%) | 318,000 (177.8) | 156,000 (99.4) | |
| Cmax | n | 61 | 7 |
| Geo-mean (CV%) | 55,700 (155.4) | 20,000 (71.6) | |
| Tlast | n | 62 | 8 |
| Median (min, max) | 102 (17.8, 380) | 27.8 (20.9, 83.9) |
Abbreviations/definitions: AUC0-28d, exposure or levels of transgene attained during the initial 28 days following infusion of tisagenlecleucel; Cmax, maximum (peak) expansion of transgene post-tisagenlecleucel infusion; CV, coefficient of variation; Responder, patient with CR or CRi; Tlast, time of last observed quantifiable transgene. Adapted from "Oncologic Drugs Advisory Committee Briefing Document: Tisagenlecleucel (CTL019)," available at https://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/oncologicdrugsadvisorycommittee/ucm566168.pdf.
A key factor in the success of CAR-T cell therapy is the ability of the infused T cells to proliferate and expand. Indeed, in the CTL019 trials, the time to peak T cell expansion was 10 days in patients who obtained a complete remission, compared with 20 days in those with no response. Both the degree of CAR-T cell expansion and the duration of transgene detection (102 vs. 28 days) were also markedly higher in patients who responded to therapy (Table). In order to encourage T cell engraftment, most patients are given a lymphodepleting conditioning therapy, which must be completed between two and 16 days prior to T cell infusion. This treatment creates “space” within the peripheral lymphoid pool, and T cells then undergo rapid expansion through the process of homeostatic proliferation. Typical regimens include fludarabine and cyclophosphamide, but there is interest in the development of experimental approaches that might omit this stage of the protocol.
The number of T cells that is infused has been a key variable in different studies. The pivotal registration trial for CTL019 used a single infusion of 0.2 to 5 million transduced viable T cells/kg for patients below 50 kg and a dose of 0.1 to 250 million T cells for those greater than this weight. As such, a typical cell dose of 100 million T cells is equivalent to the number of lymphocytes present in only 100 mL of blood. Interestingly, no association has been seen between the dose of T cells in the CTL019 trials and the degree of T cell expansion, which suggests that the optimal number of cells for infusion is currently unclear. The approach of using defined ratios of transduced CD4+ and CD8+ T cells has been associated with both robust T cell expansion and clinical response,3 and it is clear that optimization of graft engineering will play a key role in the evolution of CAR-T cell therapy.
Toxicity
Adverse effects following CAR-T cell therapy remain a significant concern. The major problem remains cytokine release syndrome (CRS), which is seen in up to 80 percent of patients and may reach grade 3/4 in half of these cases. Higher levels of disease burden and a rapid onset of symptoms are predictive of more severe effects and CRS normally lasts for around eight days. Tumour lysis syndrome is much less of a concern and is well-managed using standard approaches. Treatment algorithms for CRS can include administration of anti-interleukin (IL)-6 cytokine-directed therapy with agents such as tocilizumab and siltuximab, which are needed in around one third of cases. Support with vasopressors or ventilatory support is required for only a minority of patients.
Neurological toxicity is a perplexing and common feature of CAR-T cell therapy and includes features such as seizure, confusion, and encephalopathy. Similar features have been seen with antibodies that engage T cells, such as BiTE® (bispecific T-cell engagers; Amgen Inc.) therapy, and it is unknown if this results from cytokine release or direct T cell toxicity. These features usually resolve within the first 30 days. The long-term effects of CAR-T cells are currently uncertain. Gene therapy trials using retroviral vectors have been previously associated with secondary leukemia due to insertional mutagenesis, but the CTL019 vector incorporates a self-inactivating lentiviral vector and no such cases have been reported in CAR-T cell studies. Persistence of CAR-T cells will also suppress normal B-cell development, and prophylactic immunoglobulin infusions may often be required.
Clinical Activity
The clinical responses to therapy have been highly encouraging. Remission rates of 70 to 95 percent are typical for patients with relapsed or refractory ALL, and up to 75 percent of patients remain in remission at six months. Although the median time of follow-up remains quite short, early evidence suggests that remissions are robust, and typically around 80 percent of patients remain alive after one year of follow-up. Minimal residual disease negativity within the bone marrow, typically defined as being less than 0.01 percent, is commonly achieved. The efficacy of CAR-T cells is remarkably swift, and in patients who respond to CAR-T cell therapy, transgene levels in the bone marrow are typically at the highest level at day 28, followed by a subsequent decline, and were measurable up to six months.
The BiTE antibody blinatumomab has a defined role in the management of relapsed ALL, and the potential role of CAR-T cells must be assessed in relation to its proven utility. Currently no analysis of the two agents in a “head-to-head” clinical study has been reported, and until that time, it is impossible to assess their relative merits. However, the selection for loss of CD19 expression is a concern, and as such, it may not prove feasible to use the two approaches in a sequential manner.
Future Directions
Although CD19+ lymphoid diseases have served as the focus of CAR-T cell therapy, the approach is rapidly being applied in other disease settings. Within multiple myeloma, the field is moving rapidly, with considerable encouragement from the targeting of B-cell maturation antigen, CD138, and κ-light chain. The identification of tumor-selective targets has proven difficult in the development of CAR-T cell therapy for myeloid diseases, but proteins such as CD33 and CD123 may offer hope.
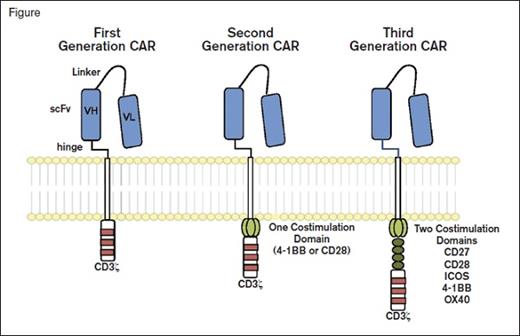
Chimeric Antigen Receptors. Chimeric antigen receptor (CAR) molecules link an extracellular single-chain variable fragment (scFv) to the CD3? intracellular domain of the T cell receptor. First-generation constructs were followed by combination with one (second generation) or two (third generation) additional costimulatory domains. From Maus MV, et al. Blood 2014; 123:2625-2635.
Chimeric Antigen Receptors. Chimeric antigen receptor (CAR) molecules link an extracellular single-chain variable fragment (scFv) to the CD3? intracellular domain of the T cell receptor. First-generation constructs were followed by combination with one (second generation) or two (third generation) additional costimulatory domains. From Maus MV, et al. Blood 2014; 123:2625-2635.
The introduction of a single transgene to encode the CAR antibody (typically anti-CD19) is only the start of our journey (Figure). A range of additional genetic modifications is being explored to drive the constitutive or inducible expression of cytokines or ligands that can extend cell survival, improve tissue localization, and even offer the opportunity to delete transfected cells if problems arise. These so-called “armored CARs” offer a glimpse into the opportunities that will arise in the next few years.
Future opportunities for CAR therapy are impressive. CTL019 has received Breakthrough Therapy designation from the U.S. FDA, which will now consider the Biologics License Application. It is now timely to suggest that all substantial hematology departments should consider the establishment of a specialist immunotherapy clinic. On our highways, the rise of autonomous cars is such that many physicians can start at least to abandon their self-driven vehicles. At a therapeutic level, the introduction of CAR-T cells threatens to be so rapid that all hematologists will soon need to learn to drive again.
Return to the Case
A leukapheresis was performed, and T cells were transfected with a lentiviral vector containing the extracellular domain of a CD19-specific antibody fused to the intracellular domain of the CD3 ζ chain. On day 0, 100 million cells were infused without complication. After 48 hours, the patient became pyrexic (40.2oC), hypotensive, and drowsy, and these symptoms of cytokine release syndrome continued despite management according to guidelines. Anti-IL6 monoclonal antibody was administered, and the symptoms came under control within four days with intensive care unit support. Laboratory analyses showed that the peripheral B cell lymphocyte count fell to less than 0.1×109/L after 10 days. A bone marrow sample obtained after three weeks showed complete remission with no detectable ALL blasts by flow cytometry. Polymerase chain reaction detection of the CD19-transgene revealed that transduced T cells were detectable for four weeks after infusion, but subsequently became undetectable. The patient was discharged from hospital within four weeks but was troubled by grade 3 neutropenia; immunoglobulin infusions were also administered due to continuing B cell aplasia. The patient remained in complete remission during follow-up at six months.
References
Competing Interests
Dr. Paul Moss is a Hematologist at Birmingham, UK, and introduced HLA-peptide-tetramer based selection of antigen-specific T cells as a novel form of T cell therapy.