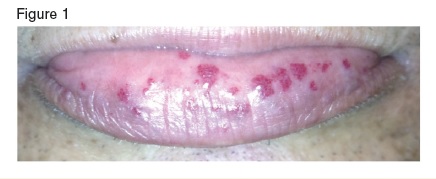
Mucocutaneous telangiectasias on the inner lower lip of a patient with hereditary hemorrhagic telangiectasia.
Mucocutaneous telangiectasias on the inner lower lip of a patient with hereditary hemorrhagic telangiectasia.
Hereditary hemorrhagic telangiectasia (HHT), also known as Osler-Weber-Rendu syndrome, is an inherited autosomal dominant disorder of vascular development characterized clinically by mucocutaneous telangiectasias (Figure 1). Patients commonly have significant epistaxis and gastrointestinal (GI) bleeding with associated iron deficiency anemia. HHT patients also develop arteriovenous malformations (AVMs) of the cerebral, pulmonary, and hepatic circulation. Mutations that alter TGF-β signaling unify the pathophysiology of the disorder: ENG and ACVRL1 that each encode TGF-β receptors, and MADH4 that encodes SMAD4, which is involved in TGF-β intracellular signaling. We present a patient with crippling hepatic AVMs who benefited from an emerging approach that employs blood vessel–modifying agents.
The Case
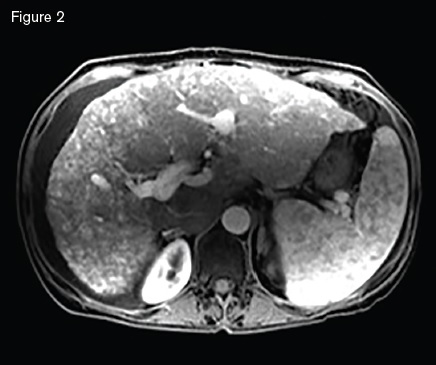
Magnetic resonance image showing a macronodular liver with diffuse arteriovenous malformations s involving both hepatic lobes, ascites, and splenomegaly.
Magnetic resonance image showing a macronodular liver with diffuse arteriovenous malformations s involving both hepatic lobes, ascites, and splenomegaly.
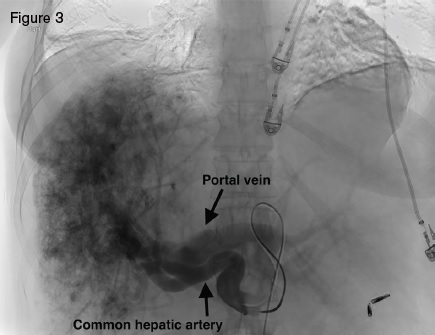
A 51-year-old woman with HHT was referred for management of recurrent epistaxis, GI bleeding, iron deficiency anemia, large bilobar hepatic AVMs, and worsening ascites. Gastric AVMs were previously managed with endoscopic argon plasma coagulation. She required regular intravenous iron and occasional blood transfusions to maintain a hemoglobin of seven to 10 g/dL. Elevated liver enzymes two years earlier prompted an ultrasound that demonstrated hepatic AVMs. Magnetic resonance imaging (Figure 2) demonstrated a macronodular liver with diffuse AVMs involving both hepatic lobes, ascites, and splenomegaly. Angiogram demonstrated large hepatic arteries with shunting primarily from hepatic artery to portal vein (Figure 3). She primarily reported weakness, dyspnea on exertion, and worsening ascites requiring weekly six- to seven-L paracentesis.
Angiogram displaying large hepatic arteries with shunting primarily from hepatic artery to portal vein.
Angiogram displaying large hepatic arteries with shunting primarily from hepatic artery to portal vein.
Hepatic AVMs and HHT
Hepatic AVMs occur in as many as 74 percent of HHT patients.1 The majority of HHT hepatic AVMs, however, are asymptomatic, making routine screening inadvisable.2 Large hepatic AVMs with extensive arterio-hepatic venous shunting can lead to high-output cardiac failure. When shunting is predominantly between the hepatic artery and portal vein, presinusoidal portal hypertension can develop, leading to ascites and nodular regenerative hyperplasia. Hepatic angiography in our patient demonstrated a large, diffuse, bilobar hepatic AVM with hepatic artery-to-portal vein shunting. The hepatic AVM was thought to be the cause of our patient’s high-output cardiac failure, portal hypertension, and debilitating ascites.
Management of Hepatic AVMs
The mainstay of management of symptomatic hepatic AVM has been liver transplantation with a reported 10-year actuarial survival of 82 percent in HHT patients.3 Other alternative treatments centered on reducing hepatic artery blood flow have been evaluated and remain controversial. Selective hepatic artery embolization has resulted in significant morbidity and mortality. The largest series of 20 patients with staged embolization using polyvinyl alcohol particles and coils reported 10 percent mortality, and 20 percent of patients developed complications including ischemic cholangitis, ischemic cholecystitis, hepatic necrosis, and abscess.4 Embolization is seldom performed at U.S. HHT centers. Recently, hepatic artery banding has been evaluated in one series with good results in which the common and either the left or right hepatic arteries are focally constricted with suture.5
Newly emerging approaches to hepatic AVMs harness the growing number of available blood vessel–modifying medications. Vascular endothelial growth factor (VEGF) is an angiogenic stimulus implicated in the pathogenesis of HHT lesions through its stimulation of TGF-β superfamily signaling. Bevacizumab, a recombinant monoclonal antibody that binds and inhibits VEGF signaling, offers a noninvasive option to treat symptomatic HHT hepatic AVMs. The largest reported prospective series investigating the use of bevacizumab comes from the French HHT Network.6 The authors reported 24 HHT patients with hepatic AVMs causing high-output cardiac failure who received bevacizumab 5 mg/kg every two weeks for a total of six doses. The study’s primary endpoint was echocardiographic decrease in cardiac output at three months after the first infusion. Of the 24 patients, three had a complete response with normalization of cardiac index, 17 had a partial response, and three had no response. The main side effect was grade 3 hypertension that was medically managed. Importantly, bevacizumab was also associated with improved epistaxis scores and quality of life.
Patient Follow-Up
Our patient was initially placed on the liver transplant list, but her model for end-stage liver disease (MELD) score of 34 kept her low on the list. Given her progressive and crippling ascites that was making her life miserable, she opted for bevacizumab therapy 5 mg/kg every two weeks (6 doses). Her weekly paracentesis diminished in volume and frequency, and at six months after initiation of therapy she no longer required paracentesis, and imaging demonstrated only trace ascites. She experienced thinning of her hair during treatment, which improved back to baseline over time. She reported no other side effects. Her epistaxis nearly completely resolved. She then received 5 mg/kg bevacizumab every two weeks for a total of four doses, which she tolerated well. With her urging, we recently initiated maintenance dosing of bevacizumab 5 mg/kg monthly for two infusions, repeating every four months. Her marked improvement moved her to status 7, temporarily inactive on the liver transplant list, and she has returned to work.
The findings with bevacizumab6 raise many exciting questions. Do we need maintenance treatment? If so, how much, at what interval, and how long? Maintenance dosing after six infusions has not been formally studied. However, some HHT centers offer maintenance therapy on a case-by-case basis using 2.5 to 5 mg/kg monthly for two to three doses with repeat cycle intervals based on team and patient shared decision-making. These decisions are devoid of clinical trial data. HHT clinicians have speculated that bevacizumab may be less effective with large caliber vascular shunts compared to AVMs with diffuse smaller vascular connection, but this also lacks good supporting data.
The introduction of blood vessel–modifying treatments marks the beginning of a new era in HHT management. It is an exciting time but largely a data-free zone. Investigations of alternative agents include thalidomide7 and pazopanib.8 However, if we are to make significant inroads, these approaches need to be studied in an orderly manner. We recommend that you refer patients to HHT Centers of Excellence (https://curehht.org/resources/hht-treatment-centers/https://curehht.org/resources/hht-treatment-centers/) or consult such centers to help guide treatment and, hopefully soon, to enroll patients in clinical trials.
References
Competing Interests
Drs. Conrad, Dickey, Hetts and Leavitt indicated no relevant conflicts of interest.