The Case
A 76-year-old woman has high-risk myelodysplastic syndrome (MDS) that was diagnosed two years ago. She initially responded to and tolerated a hypomethylating treatment, but recently developed progressive cytopenias, and her bone marrow biopsy revealed progression to acute myeloid leukemia (AML) with 25 percent blasts. After discussion with the patient, considering her preferences, functional status, and comorbidities, a decision is made not to proceed with induction chemotherapy. She is interested in pursuing treatment and has heard about clinical trials from a friend. Although several trials that are open for enrollment are identified, the patient either does not meet eligibility criteria or lives too far away from the study sites. She is informed that access to an investigational drug outside of a clinical trial might be possible, but it is not clear whether treatment can be arranged quickly.
The Question
How does one determine if access to an investigational agent outside of a clinical trial is feasible, and if so, how is access obtained for the patient?
The Response
The U.S. Food and Drug Administration (FDA) has mechanisms in place to facilitate access to investigational drugs (including biologics) for treatment use through expanded access programs, sometimes referred to as “compassionate use” programs.1 These programs were established to provide access to investigational drugs (i.e., products that have not been FDA approved) outside of clinical trials to patients with serious or immediately life-threatening diseases or conditions for whom no satisfactory alternative therapies are available. The primary purpose is to provide access rather than obtain information about the safety or efficacy of an investigational drug. Under these programs, the pharmaceutical developer also must agree to provide access to the investigational agent.
Is Expanded Access Appropriate for My Patient?
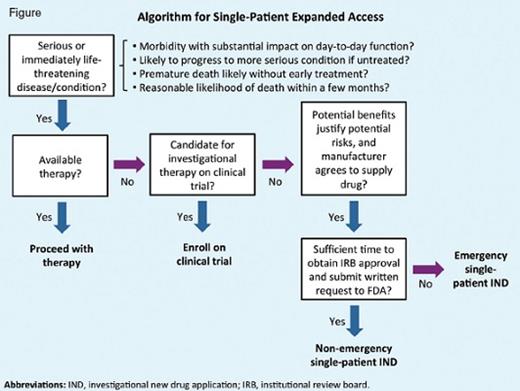
Several key criteria must be met for a patient to be eligible for expanded access (Figure).2 First, the patient must have a serious or immediately life-threatening disease or condition, based on clinical judgement. Second, it must be determined that there are no comparable or satisfactory treatments available for the patient and that they are unable to enroll in an existing clinical trial. For example, a patient may have exhausted all available therapies, have a contraindication to an approved drug, have a rare disease with no approved treatment, or fail to meet eligibility criteria for a clinical trial. In some cases, a protocol exception can be granted for a patient who otherwise does not meet eligibility criteria to allow them to receive the investigational drug as part of a clinical trial. Finally, the physician must decide whether investigational use is justified considering the potential benefits and risks.
Expanded Access Programs
Three categories of expanded access exist: 1) for individual patients, including for emergency use; 2) for intermediate-size patient populations; and 3) for widespread use in larger patient populations through an investigational new drug (IND) application (also known as a treatment IND) or treatment protocol.3 The first category is relevant to our case and is the type of expanded access program hematologists would most likely encounter.
Individual patient-expanded access is often referred to as “single-patient” expanded access. Requests for single-patient expanded access can either be added to an existing IND as a new protocol or submitted as a separate new IND. If an existing IND is used to provide access, the sponsor of that IND submits a new protocol for individual patient use to the FDA under the existing IND. Most commonly, the treating physician takes on the role of sponsor-investigator of a new single-patient IND and submits the application to the FDA. The second and third categories of expanded access are usually obtained under an existing IND sponsored by the pharmaceutical company or by another group or investigator.
Requesting Expanded Access
Before submitting a request to the FDA, practitioners should contact the drug manufacturer to confirm whether they will provide the drug for expanded access use (Figure). Although drug manufacturers are not required to supply investigational drugs outside of clinical trials, manufacturers may have established expanded access protocols in place. If there is an established expanded access program, you should follow the manufacturer’s procedures for that program. If the manufacturer agrees to provide the investigational product under a separate single-patient IND, they will provide a letter of authorization (LOA), which grants FDA authority to reference information within the manufacturer’s existing IND to fulfill some requirements for the application. The process and timelines for FDA authorization of single-patient expanded access differ for emergency versus non-emergency use.4
Requesting Emergency Use
Emergency expanded access is intended for situations when a patient requires urgent treatment before institutional review board (IRB) approval can be obtained and a written request can be submitted to the FDA (Figure). In this situation, an FDA official can grant authorization over the phone, and treatment may begin as soon as the drug is received from the manufacturer (Table). Typically, the FDA will respond within a day to requests for single-patient INDs for emergency use.5 Be prepared to provide information regarding the patient’s clinical history, including treatment history; rationale for use of the investigational drug; and proposed treatment plan, including the dose, schedule, and route of administration, planned duration of therapy, and monitoring.
Table. Process for Obtaining an Emergency Single-Patient IND
| 1. Determine whether an emergency single-patient IND is needed (see Figure) |
| 2. Contact the pharmaceutical company developing the drug (or biologic) to request permission for expanded access to the investigational agent for treatment use. |
3. Request authorization from FDA for an emergency single-patient IND.*
Be prepared to provide:
|
4. Treatment may start immediately after:
|
| 5. Notify IRB of the emergency expanded access use within 5 business days of treatment. |
| 6. Submit FDA Form 3926 (expanded access application) and a Letter of Authorization (LOA) from the drug manufacturer to FDA within 15 business days of FDA authorization. |
| 1. Determine whether an emergency single-patient IND is needed (see Figure) |
| 2. Contact the pharmaceutical company developing the drug (or biologic) to request permission for expanded access to the investigational agent for treatment use. |
3. Request authorization from FDA for an emergency single-patient IND.*
Be prepared to provide:
|
4. Treatment may start immediately after:
|
| 5. Notify IRB of the emergency expanded access use within 5 business days of treatment. |
| 6. Submit FDA Form 3926 (expanded access application) and a Letter of Authorization (LOA) from the drug manufacturer to FDA within 15 business days of FDA authorization. |
*Call FDA's Division of Drug Information (DDI) at 855-543-3784 during normal business hours (8 a.m. - 4:30 p.m. EST weekdays), or 866-300-4374 after hours.
Approval of Expanded Access Requests
Historically, the FDA has approved nearly all of the requests it has received for expanded access for treatment use.6 Recent FDA analysis showed that the Center for Drug Evaluation and Research, which includes the Office of Hematology and Oncology Products (OHOP), approved 99.7 percent of the more than 10,000 new expanded access single-patient IND requests received between 2005 and 2014.7
Steps After Expanded Access Request Is Approved
As the sponsor-investigator of a single-patient IND, you assume all responsibility for managing the use of the investigational drug. Informed consent must be obtained before starting treatment. The patient must be able to understand and willing to accept the uncertainty regarding the safety nd effectiveness of an investigational drug. After you submit a single-patient IND for nonemergency use, you must wait 30 days to begin treatment, unless earlier notification is received from the FDA. If the FDA authorizes a single-patient IND for emergency use, treatment may begin as soon as the drug is received, but you must agree to notify the IRB of the emergency expanded access use within five business days and to submit an application to the FDA within 15 business days (Table). An independent IRB may be used if you do not have access to a local IRB. Form FDA 3926 can be used to apply for single-patient expanded access, including for emergency use. You are responsible for reporting of adverse events and for appropriate monitoring throughout the course of treatment. After therapy with the investigational drug is complete, you should provide a summary of the treatment results to the FDA and may also request to withdraw the single-patient IND at that time.
In summary, while participation in a clinical trial is preferred whenever possible, expanded access programs facilitate access to investigational therapies, when appropriate, for patients without other options. Further information on expanded access for patients, physicians, and industry can be found at www.fda.gov/NewsEvents/PublicHealthFocus/ExpandedAccessCompassionateUse/default.htmwww.fda.gov/NewsEvents/PublicHealthFocus/ExpandedAccessCompassionateUse/default.htm.
References
Competing Interests
Dr. Baines and Dr. Farrell indicated no relevant conflicts of interest.