Classic Hodgkin lymphoma (cHL) is a lymphoma of B cells where the large neoplastic cells — Hodgkin Reed-Sternberg (HRS) cells — are typically rare and often surrounded by a rich inflammatory background. Yet, despite the exuberant inflammation, the lymphoma growth evades host-versus-lymphoma immune surveillance and, without therapy, is rapidly fatal. Gene expression profiling of the inflammatory cells singled out tumor-associated macrophages (TAMs) as correlating with poor outcomes.1 In 2010, Dr. Christian Steidl and colleagues reported that cases of cHL with increased TAMs (marked by CD68) had significantly worse progression-free survival in both univariate and multivariate analysis as well as with 10-year disease-specific survival (p = 0.003 for all analyses), and that this variable outperformed the International Prognostic Score.1 However, with the collective level of understanding at the time, these findings were largely phenomenological, and subsequent literature in the field provided conflicting evidence. For pathologists, after an initial flurry of CD68 stains, the practice of enumerating TAMs fell out of favor. However, Dr. Claudio Agostinelli recently found that increased CD68+ TAMs have again been implicated in poor outcomes in patients with negative fluorodeoxyglucose (FDG) -PET scans after two courses of ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine).2
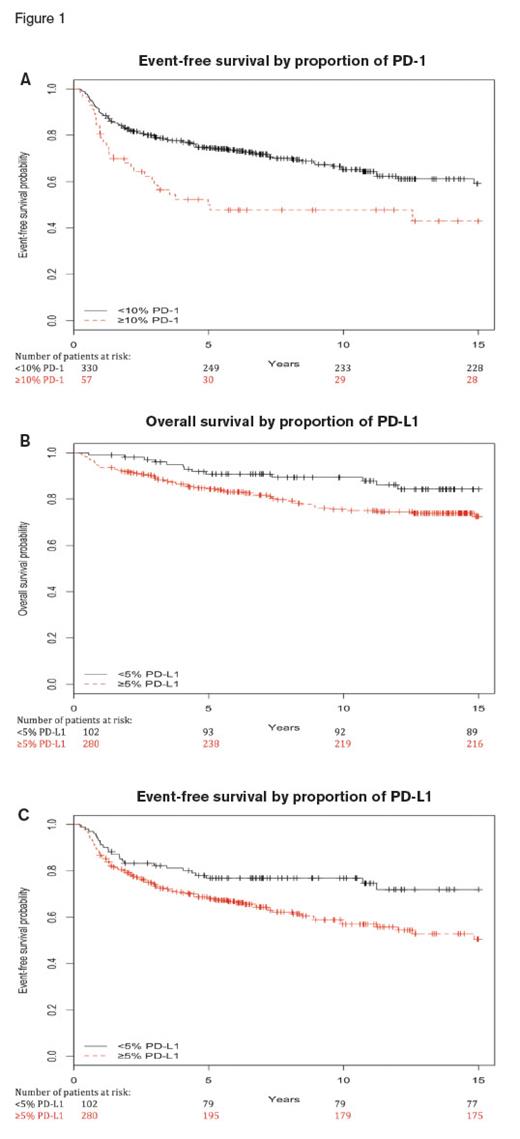
(A) Kaplan-Meier plots of event free survival of cases with 10 percent or greater (red dashed line) and less than 10 percent (blue solid line) PD-1 expression. (B) Kaplan-Meier plots of event-free survival of cases with 5 percent or greater (red dashed line) and less than 5 percent (blue solid line) PD-L1 expression. (C) Kaplan-Meier plots of overall survival of cases with 5 percent or greater (red dashed line) and less than 5 percent (blue solid line) PD-L1 expression. Adapted from Hollander P., et al. Blood Adv. 2017;1:1427-1439.
(A) Kaplan-Meier plots of event free survival of cases with 10 percent or greater (red dashed line) and less than 10 percent (blue solid line) PD-1 expression. (B) Kaplan-Meier plots of event-free survival of cases with 5 percent or greater (red dashed line) and less than 5 percent (blue solid line) PD-L1 expression. (C) Kaplan-Meier plots of overall survival of cases with 5 percent or greater (red dashed line) and less than 5 percent (blue solid line) PD-L1 expression. Adapted from Hollander P., et al. Blood Adv. 2017;1:1427-1439.
Two recent papers have clarified the role of inflammation, including TAMs, in prognosis of cHL. In the first article, Dr. Peter Hollander and colleagues studied the expression of PD-1 and PD-L1 in the microenvironment of cHL and found inferior event-free survival (EFS) in patients with high levels of expression of these markers with conventional chemotherapy regimens. They found that patients with high levels of PD-1 expression on lymphocytes and monocytes (≥10% positive cells, as determined by digital image analysis of immunohistochemical stains) had shorter EFS compared with those who had low expression (p=0.002; univariate hazard ratio, 1.91; multivariate p=0.02; Figure 1A). Similarly, patients with high levels of PD-L1 (≥5% positive cells) had shorter EFS compared with those who had low expression (p=0.01; univariate hazard ratio, 1.74; multivariate p=0.03) and shorter overall survival (multivariate p=0.03; Figure 1B and 1C). Although this study limited their assessment of the tumor microenvironment to background lymphocytes and monocytes (excluding HRS cells, granulocytes, and TAMs), the authors acknowledged that the poor outcomes associated with PD-L1 expression might be in part due to PD-L1–positive macrophages.
(A) Cellular phenotype map depicting locations of PD-L1+ Hodgkin Reed-Stemberg (HRS) cells (orange dots), PD-L1+ tumor-associated macrophages (TAMs) (purple dots), and PD-L1- TAMs (pink dots). (B) Representative image (40x resolution) showing CD4+ T cells (left panel, green) with coexpression of PD-1 (right panel, yellow) touching CD68+ TAMs (both panels, magenta). Adapted from Carey CD, et al. Blood. 2017;130:2420-2430.
(A) Cellular phenotype map depicting locations of PD-L1+ Hodgkin Reed-Stemberg (HRS) cells (orange dots), PD-L1+ tumor-associated macrophages (TAMs) (purple dots), and PD-L1- TAMs (pink dots). (B) Representative image (40x resolution) showing CD4+ T cells (left panel, green) with coexpression of PD-1 (right panel, yellow) touching CD68+ TAMs (both panels, magenta). Adapted from Carey CD, et al. Blood. 2017;130:2420-2430.
In a second article, Dr. Christopher D. Carey and colleagues specifically identified PD-1+ lymphocytes and PD-L1+ macrophages and determined their spatial relationship to the HRS cells in an elegant study using multiplexed immunofluorescence and digital image analysis. They visualized and quantitated the enrichment of PD-L1+ TAMs in proximity to HRS cells compared with PD-L1- TAMs (p <0.001; Figure 2A). Additionally, they found enrichment of CD4+/PD-1+ T cells in proximity to, and directly contacting, both HRS cells (p <0.01 for increased proportion of cells in physical interaction with HRS cells) and PD-L1+ TAMs (p=0.004; Figure 2B).
From Carey CD, et al. Blood. 2017;130:2420-2430. IFNγ, interferon γ.
From Carey CD, et al. Blood. 2017;130:2420-2430. IFNγ, interferon γ.
The findings by Dr. Carey and colleagues create a vivid image of the relationships within the tumor microenvironment of cHL with PD-L1+ HRS cells both directly interacting with PD-1+/CD4+ T cells and recruiting their own flanking guard of PD-L1+ TAMs that further interact with PD-1+/CD4+ T cells (Figure 3). Extensive PD-L1:PD-1 engagement in the immediate vicinity of HRS cells defines an “immunoprotective niche” to suppress T cell activation and effective antitumor immunity.
These results help explain the high overall response rates to PD-1 blockade strategies in relapsed/refractory cHL, with 66.3 percent objective response rates (ORR) to nivolumab and 69.0 percent ORR to pembrolizumab.3,4 In the case of nivolumab, response was correlated with PD-L1 expression in HRS cells.3 The findings of Dr. Carey and colleagues suggest that the correlation with PD-L1 expression on TAMs should be examined as well.
In Brief
These studies illuminate the etiology of the previously reported association between high macrophage counts and poor response to various traditional chemotherapy regimens, as well as the remarkable responses to PD-L1–directed immunotherapy in relapsed/refractory cHL. TAMs surround the HRS cells and interact closely with CD4-positive T cells to shield the HRS cells, effecting a Jedi mind trick on the immune system (“these are not the HRS cells you are looking for...”). These findings support an increased role of PD-1 blockade strategies in the treatment of cHL. Additionally, this study reiterates the need for pathologists to enumerate TAMs and their PD-L1 expression in cases of cHL for prognostic and immunotherapeutic purposes.
References
Competing Interests
Dr. Kim indicated no relevant conflicts of interest.