FLT3-ITD–mutant acute myeloid leukemia (AML) is associated with a high rate of relapse postallograft, and outcomes for patients who relapse are very poor.1 Empirically, this has prompted trials of “maintenance therapy” with inhibitors of FLT3 such as sorafenib following allogeneic transplantation. While the jury may still be out on whether such therapy should be routine, some patients who relapse postallograft can be rescued with sorafenib therapy if there is induction of graft-versus-host disease (GVHD) and associated graft-versus-leukemia (GVL). Having evidence to explain how responses and apparent cures are achieved and why only some patients benefit would certainly strengthen the case for further exploration of this approach.
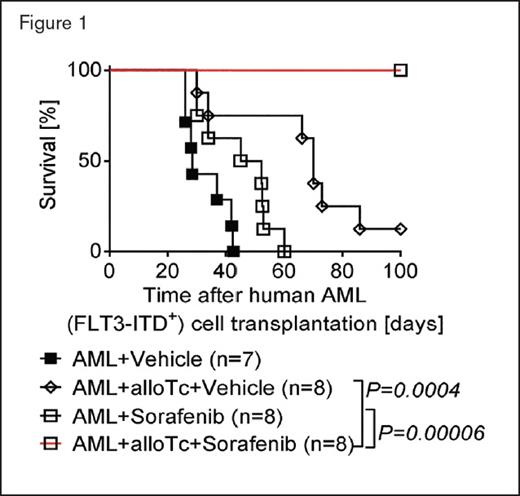
Percentage survival of NSG mice injected with primary human FLT3-ITD+ acute myeloid leukemia (AML) cells derived from an HLA-A2+ patient with or without additional allogeneic human CD8+ T cells that had been stimulated and expanded in the presence of autologous dendritic cells expressing allogeneic HLA-A2 upon RNA transfection in vitro. Mice were treated with vehicle or sorafenib as indicated. The experiment was performed twice with similar results; n values represent biologically independent mice. p values were calculated using the two-sided Mantel-Cox test.
Percentage survival of NSG mice injected with primary human FLT3-ITD+ acute myeloid leukemia (AML) cells derived from an HLA-A2+ patient with or without additional allogeneic human CD8+ T cells that had been stimulated and expanded in the presence of autologous dendritic cells expressing allogeneic HLA-A2 upon RNA transfection in vitro. Mice were treated with vehicle or sorafenib as indicated. The experiment was performed twice with similar results; n values represent biologically independent mice. p values were calculated using the two-sided Mantel-Cox test.
Dr. Nimitha R. Mathew and colleagues have sought to understand how treatment with sorafenib may trigger an allo-immune response. Using sophisticated mouse models and primary samples from patients in vitro and in vivo, they dissected how each of the constituent elements of the system (targeted inhibitor, T cells, cytokines, AML cells, and FLT3-ITD mutation) contribute to leukemia control in an allograft setting. First, in a model setting, they demonstrated that long-term control of leukemia postallograft requires both treatment with sorafenib and allogeneic T cells — neither was effective alone, nor was the combination of sorafenib with syngeneic T cells. This dual requirement was consistently observed in several models, including a humanized model using primary human FLT3-ITD–mutated AML (Figure 1). Furthermore, the cure of leukemia could occur without life-threatening GVHD. Second, this effect was driven specifically by increased IL-15 production, with increased levels observed in sera. When IL-15 was blocked, no antileukemic effect was observed, and sorafenib did not directly influence production of other cytokines. Importantly, the increased IL-15 production was from the FLT3-ITD–mutant AML cells, not from the donor cells. While systemic treatment with exogenous IL-15 could replace the production from leukemia cells, this resulted in severe GVHD and increased lethality. The authors suggest that as the GVL effect extinguishes the AML, the source of the instigating IL-15 production is removed, eliminating a driver for unrestrained GVHD. Third, CD8+ T cells were the effector cells for the allo-immune response. While sorafenib had no direct effect on T cell activation in vitro, sorafenib-treated AML-bearing mice generated more CD8+ CD107a+ IFN-γ+ T cells, which could impart long-term leukemia control if transplanted into secondary recipients with the same leukemia (but not a third-party leukemia). Fourth, this sorafenib-induced allo-immune response was not observed in models where AML lacked the FLT3-ITD mutation. Indeed, when AML cells were engineered with a variant of FLT3-ITD that was resistant to sorafenib inhibition, no sorafenib-induced IL-15 production or leukemic protection was observed.
IL-15 levels in the serum of patients who relapsed with FLT3-ITD+ AML after allo-HCT. Sorafenib–DLI responders and nonresponders are shown separately. Each data point represents the IL-15 level in the serum of a patient before sorafenib (so.) treatment (day 0) and after the start of sorafenib treatment (day 3; before the patients received donor lymphocyte infusions). The dashed line indicates the detection limit (4 pg/mL) of the IL-15 ELISA. n values represent biologically independent patients. p value was determined using the twosided Wilcoxon matched-pairs signed-rank test.
IL-15 levels in the serum of patients who relapsed with FLT3-ITD+ AML after allo-HCT. Sorafenib–DLI responders and nonresponders are shown separately. Each data point represents the IL-15 level in the serum of a patient before sorafenib (so.) treatment (day 0) and after the start of sorafenib treatment (day 3; before the patients received donor lymphocyte infusions). The dashed line indicates the detection limit (4 pg/mL) of the IL-15 ELISA. n values represent biologically independent patients. p value was determined using the twosided Wilcoxon matched-pairs signed-rank test.
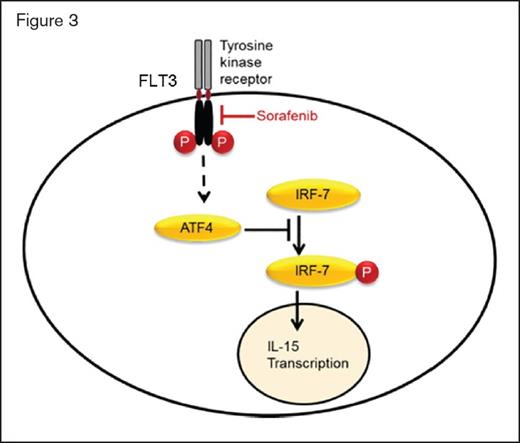
Consistent with these model findings, when patients with FLT3-ITD–mutant AML relapse postallograft were treated with sorafenib and donor lymphocyte infusion (DLI), serum IL-15 and IFN-γ levels at day 6 of sorafenib therapy were only increased in patients who ultimately demonstrated a clinical response (Figure 2). The investigators elegantly demonstrated that induction of IL-15 production required sorafenib-induced inhibition of mutant FLT3-ITD. Constitutive signalling from the mutant receptor leads to production of the transcription factor ATF4. Inhibition of FLT3-ITD signalling reduces ATF4 levels, which then reduces repression of IRF-7 activation. Increased IRF-7 activation in turn increases IL-15 transcription (Figure 3). When FLT3-ITD is resistant to sorafenib, there is no change in ATF4 levels, IRF-7 activation, or IL-15 production by the leukemia cells, and hence, no downstream allo-immune effect and no clinical response. Finally, while sorafenib can inhibit other kinases, this effect was specific to its ability to inhibit FLT3 and was recapitulated by other more specific FLT3 inhibitors.
Sorafenib inhibits FLT3 receptor tyrosine kinase signalling, which normally leads to ATF4 production. Reduced ATF4 levels result in less inhibition of IRF7 phosphorylation and activation. Active p-IRF7 can translocate to the nucleus, where it activates IL-15 transcription.
Sorafenib inhibits FLT3 receptor tyrosine kinase signalling, which normally leads to ATF4 production. Reduced ATF4 levels result in less inhibition of IRF7 phosphorylation and activation. Active p-IRF7 can translocate to the nucleus, where it activates IL-15 transcription.
In Brief
In summary, Dr. Mathew and colleagues have convincingly elucidated a mechanism by which a small molecule inhibitor targeting a leukemia-specific kinase mutation can trigger an effective CD8 T cell–mediated allo-immune response that leads to durable leukemia control. The detailed experiments conducted by these investigators highlight that the clinical responses observed with DLI and sorafenib are probably not due to nonspecific allo-immune responses or due to direct cytotoxicity by sorafenib. Rather, they reflect specific inhibition of FLT3-ITD with consequent upregulation of IL-15 production by the leukemia cells themselves, which then fuels a GVL effect with immune memory. With ongoing randomized studies of planned maintenance therapy with FLT3 inhibitors postallograft for patients in remission (as opposed to relapsed disease), it is interesting to speculate as to the mechanism by which these drugs may add benefit; will it be via a direct cytotoxic effect or by enhancing GVL? The discoveries described in this article provide strong support for ongoing efforts to advance the DLI-sorafenib combination and similar combinations with other FLT3 inhibitors as a therapeutic option. This new concept also highlights the importance for hematologists to understand how targeted agents are working when they are combined with immunotherapy.
References
Competing Interests
Dr. Roberts indicated no relevant conflicts of interest.