Study Title:
Impact of Home-based Oxygen Therapy on Maternal and Fetal Complications, in Pregnant Women With Sickle Cell Disease. A Randomized Multi-center Trial.
ClinicalTrials.gov Identifier:
Participating Centers:
Hôpital Necker Enfants-Malades (Public Hospitals of Paris) and nine French hospitals
Accrual Goal:
200 participants
Study Design:
This is an open-label, randomized, multicenter trial. Participants, pregnant women with sickle cell disease (SCD), are randomly assigned to home-based oxygen therapy or no home-based oxygen therapy. Primary and secondary measures will include occurrence of at least one vaso-occlusive complication lasting more than 24 hours during pregnancy and 30 days postpartum, respectively. Other secondary outcome measures include, but are not limited to, pregnancy-induced hypertension, pre-eclampsia, eclampsia, and perinatal and neonatal death rates. A vaso-occlusive event is defined as pain in the bones, acute chest syndrome, ischemic stroke, cardiomyopathy, pulmonary hypertension, splenic and hepatic sequestration, and death of the mother. A total of 200 women are expected to be enrolled throughout a course of six years, with expected trial completion in February 2021.
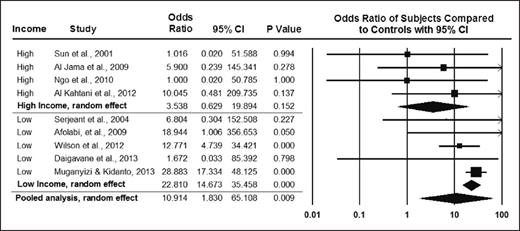
From BJOG, Boafor TK et al, Pregnancy outcomes in women with sickle-cell disease in low and high income countries: a systematic review and meta-analysis, Vol 123, 691-698. Copyright © 2016 by John Wiley Sons, Inc. Reprinted by permission of John Wiley Sons, Inc.
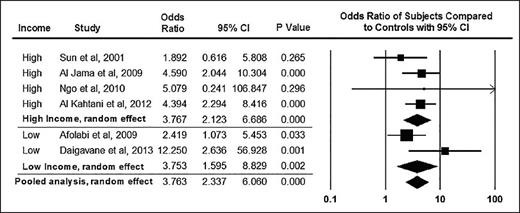
From BJOG, Boafor TK et al, Pregnancy outcomes in women with sickle-cell disease in low and high income countries: a systematic review and meta-analysis, Vol 123, 691-698. Copyright © 2016 by John Wiley Sons, Inc. Reprinted by permission of John Wiley Sons, Inc.
Rationale:
As survival for women with SCD continues to improve in both high- and low-resource settings, researchers expect that more women with SCD will become pregnant and thus susceptible to adverse obstetric events and SCD-related complications during pregnancy. Maternal and newborn complications are well recognized in pregnant women with SCD. A pooled analysis of pregnant women with SCD indicated that maternal and perinatal mortality odds ratios (ORs) were significantly higher compared with those of women without SCD (OR, 10.91 [95% CI, 1.83-65.11] vs. 3.76 [95% CI, 2.34-6.06], respectively1 ; Figures 1 and 2). Pregnant women with SCD have higher rates of adverse maternal and fetal complications including, but not limited to, pre-eclampsia and intrauterine growth restriction (pooled OR, 2.05 [95% CI, 1.47-2.85] vs. 2.79 [95% CI, 1.85-4.21], respectively). Few randomized controlled trials on preventing these adverse events in pregnant women have been conducted, and no evidence-based strategy has emerged as an effective intervention to prevent these life-threatening and life-alternating complications of pregnancy in women with SCD.
From BJOG, Boafor TK et al, Pregnancy outcomes in women with sickle-cell disease in low and high income countries: a systematic review and meta-analysis, Vol 123, 691-698. Copyright © 2016 by John Wiley Sons, Inc. Reprinted by permission of John Wiley Sons, Inc.
From BJOG, Boafor TK et al, Pregnancy outcomes in women with sickle-cell disease in low and high income countries: a systematic review and meta-analysis, Vol 123, 691-698. Copyright © 2016 by John Wiley Sons, Inc. Reprinted by permission of John Wiley Sons, Inc.
Comment:
Oxygen trials in SCD are not new. One randomized controlled trial compared overnight autoadjusting continuous airway pressure plus standard care, versus standard care alone for six months, resulting in improved cognitive morbidity.2 The phase II trial in children and adults with SCD was based on an early safety trial showing no adverse events associated with the administration of oxygen for six weeks in children with SCD.3 The primary outcome is the cancellation subtest from the Wechsler scales. Secondary outcomes include, but are not limited to, general cognitive functioning, quantitative brain MRI, quality of life, and daily pain via a smartphone App (GoMedSolutions, Inc).
The biological premise for both the obstetric and cognitive trials is that improvement in nocturnal oxygen saturation will have significant increase in oxygen delivery and will improve the clinical outcomes of interest. An alternative strategy, and perhaps complementary strategy to improve oxygen delivery to vital organs, includes therapeutic agents that will increase baseline hemoglobin levels such as regular blood transfusion, hydroxyurea, or other investigative agents. Conceivably these therapeutic agents would have a more sustained impact than nocturnal oxygen supplementation or overnight autoadjusting continuous airway pressure. However, these have not been tested in pregnancy because pregnant women typically are excluded from therapeutic trials that are intended to increase baseline hemoglobin levels.
Unfortunately, the results of DR02G will not be transferable to at least 90 percent of the pregnant women with SCD living in lower middle-resource settings. Alternative strategies will need to be considered for this population. Regardless of the results of DR02G, the SCD community will anxiously await completion of the trial as it will provide important prospectively and rigorously obtained estimates for adverse obstetric and perinatal related outcome measures for women with SCD living in high-income settings. The investigative team is to be congratulated for addressing an emerging clinical challenge for young women with SCD wanting to start their family.
References
Competing Interests
Dr. DeBaun indicated no relevant conflicts of interest.