In the past 70 years, the survival for children and young adults with acute lymphoblastic leukemia (ALL) has improved dramatically. Historically, outcomes for patients with T-cell ALL (T-ALL) have been inferior to those with B-cell ALL (B-ALL). The difference in survival is largely driven by the higher frequency of low-risk biologic subsets in B-ALL such as ETV6-RUNX1 that are not seen in T-ALL. Moreover, patients with T-ALL tend to have higher-risk clinical features, including the presence of extramedullary disease. The salvage rate for patients with relapsed T-ALL is dismal. Accordingly, the primary goal in T-ALL is to prevent relapse through optimization of therapy in de novo patients. Unlike patients with relapsed B-ALL, who now have access to numerous highly effective novel immunotherapies, no effective immunotherapy has been translated to the clinic for T-ALL. Thus, more traditional approaches incorporating different cytotoxic chemotherapy regimens are the cornerstone of recently completed and ongoing cooperative group trials.
AALL0434 was a Children’s Oncology Group (COG) –initiated phase III randomized clinical trial that used 2×2 random assignment, comparing two different interim maintenance phases with or without six five-day courses of the antimetabolite nelarabine. Dr. Stuart S. Winter, Dr. Kimberly P. Dunsmore, and their colleagues recently reported the outcomes for the interim maintenance randomization that compared Capizzi-style escalating methotrexate (MTX) with pegaspargase (C-MTX) versus high-dose MTX (HD-MTX) in the Journal of Clinical Oncology. The group also presented the results of the nelarabine randomization at the 2018 American Society of Clinical Oncology annual meeting, demonstrating improved disease-free survival on the nelarabine arm; however, these results have not yet been published.1
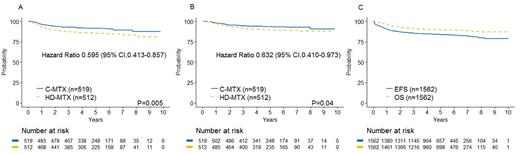
From Winter S, et al: J Clin Oncol doi: 10.1200/JCO.2018.77.7250. Reprinted with permission. © 2018 American Society of Clinical Oncology. All rights reserved.
From Winter S, et al: J Clin Oncol doi: 10.1200/JCO.2018.77.7250. Reprinted with permission. © 2018 American Society of Clinical Oncology. All rights reserved.
The large study being presented here, AALL0434, enrolled 1,895 patients aged one to 30 years with T-ALL and T-cell lymphoblastic lymphoma (T-LL) between 2007 and 2014. Patients with T-LL were not included in the interim maintenance randomization. Consent had two stages — the first for induction therapy and the second for the randomized questions. Patients with induction failure (M3 marrow; ≥ 25% blasts by morphology) or overt central nervous system (CNS) leukemia (CNS3) were non-randomly assigned to receive HD-MTX. A total of 1,031 patients with T-ALL participated in the interim maintenance random assignment. The five-year disease-free survival and overall survival (OS) rates were 91.5 percent (95% CI, 88.1% to 94.8%) and 93.7 percent (95% CI, 90.8% to 96.6%) for C-MTX, and 85.3 percent (95% CI, 81.0% to 89.5%) and 89.4 percent (95% CI, 85.7% to 93.2%) for HDMTX, respectively (Figure, parts A and B).
AALL0434 was remarkable for several reasons. First, the outcomes seen on this trial were markedly superior to those found on any trial for children and young adults with T-ALL. The five-year event-free survival (EFS) and OS rates for all T-ALL patients on the trial were 83.8 percent (95% CI, 81.2% to 86.4%) and 89.5 percent (95% CI, 87.4% to 91.7%), respectively (Figure, part C). Second, the study answered two randomized questions, demonstrating superiority for C-MTX and nelarabine, in effect establishing both now as the standard of care for children and young adults with T-ALL. While nelarabine is sometimes called a “new agent,” it was synthesized by Gertrude Elion in the 1970s. In an era of small molecule inhibitors and breakthrough immunotherapies, survival for patients with T-ALL has improved markedly as a result of thoughtfully designed randomized trials using tried-and-true agents of the past. Arguably, reshuffling standard cytotoxics may not lead to further improvements in outcome for T-ALL, but the outstanding results observed on AALL0434 are a testament to the importance of phase III randomized cooperative group clinical trials.
HD-MTX was initially hypothesized to be superior to C-MTX. A similar COG trial, AALL0232, supported superior efficacy of HD-MTX versus C-MTX in high-risk B-ALL.2 Despite opposite results, a reduction in marrow and CNS relapses was seen on the more effective arm on both trials. The AALL0434 investigators highlight that the randomization is not only a direct comparison of different schedules and doses of MTX. The HD-MTX interim maintenance included approximately two months of mercaptopurine, and prophylactic cranial radiation (pCRT) was given in delayed intensification. The C-MTX interim maintenance included two extra doses of pegasparagase, an extra dose of vincristine, and pCRT was given in consolidation approximately five months earlier in therapy. Ninety percent of patients on AALL0434 received pCRT. The investigators appropriately point out that the differences in timing of pCRT could have also contributed to the differences in outcomes. In this author’s opinion, this is unlikely. In 2016, 10 international pediatric cooperative groups pooled data on 16,623 patients with childhood ALL to assess the impact of CRT on relapse.3 Only patients with overt CNS leukemia (CNS3) had a suggested reduced risk of relapse. The addition of pCRT does not appear to improve survival or reduce relapse for T-ALL, suggesting delivery of pCRT five months earlier in therapy would not significantly improve EFS and OS.
Most international pediatric cooperative groups, including the COG, no longer give pCRT to most patients with T-ALL. On AALL1231, the successor study to AALL0434, less than 10 percent of patients receive CRT. The COG made the decision to eliminate radiation in most T-ALL patients based on results from compelling European cooperative group trials that demonstrated dexamethasone is superior to prednisone in T-ALL.4,5 AALL0434 had a prednisone-based backbone, whereas AALL1231 has a dexamethasone-based backbone. In theory, the results of AALL0434 might have been different with a dexamethasone-based backbone that did not include CRT for most patients. As it took more than a decade from design to maturation of results on AALL0434, revisiting the same randomized questions on a different backbone is likely not feasible.
In Brief
In summary, the outstanding outcomes seen for children and young adults with T-ALL on AALL0434 have raised the bar and established a new standard of care. Outcomes for childhood T-ALL are no longer inferior to those seen in B-ALL. The publication of the results of the nelarabine randomization and outcome for T-LL patients on AALL0434 is eagerly awaited.
References
Competing Interests
Dr. Teachey indicated no relevant conflicts of interest.