If something is missing, it should be replaced. Such has been the philosophy of the management of hemophilia A ever since factor VIII deficiency was first defined. Incrementally and notwithstanding substantial setbacks, progress has been made through the use of plasma products, concentrates, and recombinant factor VIII. A transition from reactive treatment of bleeding episodes to the use of prophylactic therapy that seeks to minimize the number and severity of bleeding episodes has also been developed and aims to prevent complications such as chronic joint disease. Many older hematologists will remember that hemophilia ward rounds would routinely take a detour to the orthopedic department, but this is now, thankfully, uncommon.
An alternative approach to direct replacement is to mimic the function of factor VIII, which is a cofactor for activated factor IX (IXa). In the presence of Ca2+ and phospholipids, it forms a complex that leads to the activation of factor X. Emicizumab is a bi-specific antibody that has been designed to crosslink activated factor IX and factor X, bringing them into close apposition and leading to the activation of factor X, even in the absence of factor VIII. The antibody has now been assessed in a series of stunning clinical studies throughout the past two years.
This Diffusion describes the HAVEN 3 study, which focuses on the activity of prophylactic emicizumab in patients with hemophilia who do not have inhibitors. The recent article by Dr. Johnny Mahlangu and colleagues is the third in a series on emicizumab published in the New England Journal of Medicine, with previous reports of the phase I study1 and outstanding clinical outcomes in patients who do have inhibitors.2
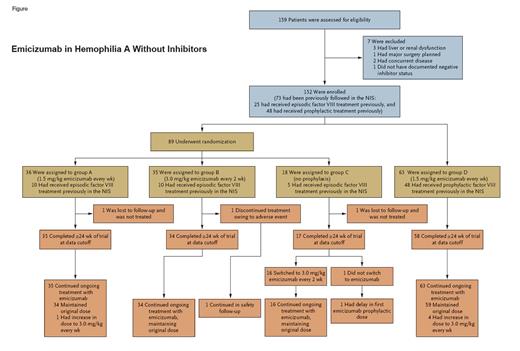
The randomized phase III HAVEN 3 study recruited 152 patients older than 12 years with severe congenital hemophilia A and endogenous factor VIII activity less than 1 IU/dL. All entrants were screened for the absence of inhibitors prior to entry (<0.6 Bethesda units/mL). Most patients were not on prophylactic factor VIII therapy, and these 89 patients were randomized in a 2:2:1 ratio to either a maintenance dose of emicizumab at 1.5 mg/kg per week (group A), 3.0 mg/kg every two weeks (group B), or no prophylaxis (group C). Sixty-three patients were already on prophylactic factor VIII infusions; in this case, emicizumab was given at a maintenance dose of 1.5 mg/kg per week, and intraindividual comparisons were made from before and after administration (group D). Prophylactic factor VIII infusions could be stopped in this group after the second dose of emicizumab had been given.
The dosing administration of emicizumab is straightforward. Four initial loading doses of 3 mg/kg are given weekly, then prophylaxis continues with either 1.5 mg/kg weekly or 3 mg/kg every two weeks. The half-life of the antibody is 30 days, which contrasts notably with the value of eight to 12 hours for factor VIII. It is important to remember that emicizumab is a prophylactic therapy, and as such, Factor VIII was given as required for breakthrough bleeding events.
The primary end-point was beautifully simple and measured the difference in the rate of treated bleeding episodes (referred to as the “annualized bleeding rate”) between the three groups. The results were truly remarkable. The annualized bleeding rate was 38.2 events in group C (no prophylaxis,) but was reduced to only 1.5 and 1.3 events in groups A and B. This equates to a 96 percent and 97 percent reduction, respectively. All patients in group C experienced at least one bleeding event, whereas 56 percent and 60 percent of those in groups A and B, respectively, did not have a single bleeding event, and more than 90 percent had at most three such events.
Outcomes in group D, in which patients had previously taken prophylactic factor VIII therapy, revealed an annualized bleeding rate of 1.6, and 56 percent of patients became entirely free of bleeding episodes. This was a particularly interesting group because the investigators could compare the annualized bleeding rate to that seen when patients were on prior prophylactic factor VIII therapy. This value was estimated at 4.8 events per year, showing that emicizumab led to a 68 percent reduction in bleeding episodes.
One of the huge advantages of emicizumab is that it is given subcutaneously every seven or 14 days, which is convenient for patient delivery. Remarkably, 94 percent of patients chose emicizumab therapy above traditional management with factor VIII. Of the 46 patients in group D who had had experience of both prophylactic factor VIII and emicizumab, all but one patient preferred emicizumab.
Treatment was well tolerated, and adverse effects were largely minimal and mainly related to injection site reactions, with only one patient stopping treatment due to factors that were thought to be related to the drug. There has been some concern that emicizumab can provoke thrombotic reactions in patients undergoing concurrent treatment with activated prothrombin complex concentrate (APCC), but no microangiopathic or thrombotic microangiopathy events were observed in this trial. This observation does have a logical explanation as factor VIII and emicizumab compete for their activity and do not have “additive” coagulation potential, whereas the combination of emicizumab and APCC could have synergistic activity in thrombin generation.
Figure showing steps taken and information on emicizumab in hemophilia A without inhibitors during the HAVEN 3 study
Emicizumab in hemophilia A without inhibitors.
Emicizumab in hemophilia A without inhibitors.
In Brief
The U.S. Food and Drug Administration has already granted breakthrough therapy designation to emicizumab for the treatment of patients with hemophilia A without factor VIII inhibitors, on the basis of the HAVEN 3 trial. Emicizumab is emerging as a genuine game changer in the treatment of hemophilia A and may come to represent a standard form of prophylaxis, with a subsequent huge reduction in usage of factor VIII. Ironically, its major threat may ultimately come from the introduction of gene therapy to “cure” the condition. These findings are evidence, indeed, of a truly remarkable transition in the management of this disorder.
References
Competing Interests
Dr. Moss indicated no relevant conflicts of interest.