The potential role of thromboprophylaxis in ambulatory patients with cancer has long been an area of active investigation. In 2016, a meta-analysis of 26 randomized controlled trials concluded that low-molecular-weight heparin (LMWH) thromboprophylaxis resulted in a modest reduction in the incidence of symptomatic venous thromboembolism (VTE), though it was associated with a nonsignificant increased risk of major bleeding.1 Despite this potential advantage, the use of thromboprophylaxis has not been widely adopted for multiple reasons, including the small increment of benefit, the burden and high cost of LMWH injections, and concerns for bleeding risk.
In the New England Journal of Medicine on December 4, 2018, the AVERT (Apixaban for the Prevention of Venous Thromboembolism in High-Risk Ambulatory Cancer Patients) investigators published the results of a novel approach to thromboprophylaxis in ambulatory patients with cancer. First, instead of LMWH, a reduced-dose direct oral anticoagulant, apixaban 2.5 mg twice daily, was used. Second, patients were selected using the Khorana score, a validated tool to identify patients at highest risk for VTE.2 Study participation required a Khorana score of 2 or higher, correlating with an estimated risk of symptomatic thrombosis of 9.6 percent for a score of 2, and 17.7 percent for a score of 3 or higher during the first six months of chemotherapy.3 Individuals with myeloproliferative neoplasms or acute leukemia were excluded. Approximately one quarter of the patients carried a diagnosis of lymphoma or multiple myeloma. A total of 574 patients were randomly assigned to receive thromboprophylaxis with apixaban or placebo, starting within 24 hours of initiation of chemotherapy and continuing for 180 days. A high–VTE-risk malignancy, as defined by Khorana score (e.g., pancreatic, gastric, or brain tumor), was present in 25.4 percent of patients; an intermediate–VTE-risk malignancy (e.g., bladder, lung, testicular, gynecologic, renal cancer, lymphoma, or multiple myeloma) was present in at least 22.1 percent of patients. The primary efficacy endpoint was objectively documented major VTE (proximal deep vein thrombosis [DVT] or pulmonary embolism [PE], either symptomatic or asymptomatic/incidentally discovered). The main safety outcome was major bleeding.
There was a significantly lower rate of VTE in the apixaban group (4.2%) compared to the placebo group (10.2%) (HR 0.41; 95% CI 0.26-0.65; p<0.001), with a number of 17 needed to treat. The rate of DVT was 2.4 percent in the apixaban group and 4.4 percent in the placebo group, and the rate of PE was 1.7 percent and 5.8 percent, respectively.
The risk of major bleeding was higher in the apixaban group, occurring in 3.5 percent of patients compared to 1.8 percent in the placebo group (HR, 2.00; 95% CI, 1.01-3.95; p=0.046), with a number of 59 needed to harm. The rates of severe (category 3 or 4) major bleeding and clinically relevant nonmajor bleeding were similar in both groups. There was no significant difference in mortality between the two groups (12.2% in the apixaban group, 9.8% in the placebo group; HR, 1.29; 95% CI, 0.98-1.71).
The AVERT trial appropriately concluded that in ambulatory patients with cancer with an increased VTE risk, thromboprophylaxis with apixaban 2.5 mg twice daily initiated at the time of chemotherapy significantly decreases the rate of VTE, with an associated comparatively smaller increase in risk of major bleeding.
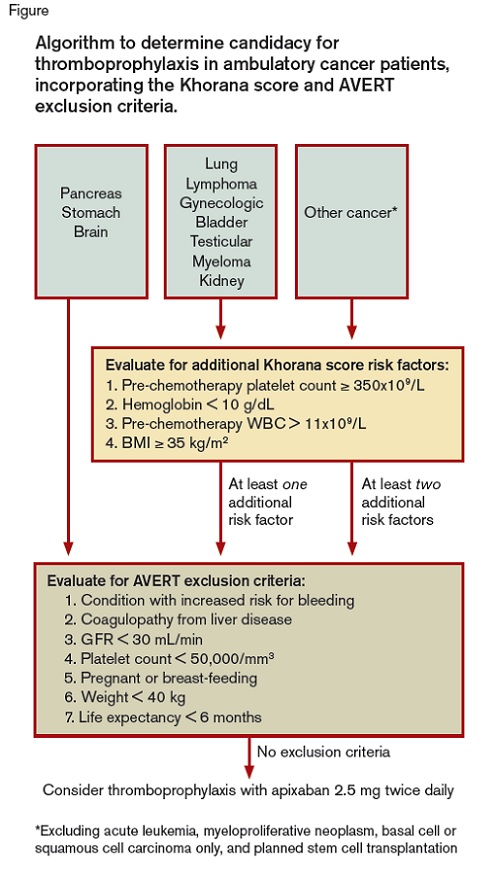
A schematic showing the algorithm used to determine candidacy for thromboprophylaxis in ambulatory cancer patients, incorporating the Khorana score and AVERT exclusion criteria
Algorithm to determine candidacy for thromboprophylaxis in ambulatory cancer patients, incorporating the Khorana score and AVERT exclusion criteria.
Algorithm to determine candidacy for thromboprophylaxis in ambulatory cancer patients, incorporating the Khorana score and AVERT exclusion criteria.
As providers question whether and how to incorporate thromboprophylaxis into their practice, it could be helpful to consider the patient population included in the AVERT trial. For assessment of study enrollment eligibility, the Khorana score was first used, followed by consideration of the AVERT trial exclusion criteria (Figure). As shown in the Figure, patients’ primary malignancy is a significant determinant of their Khorana score — those with pancreatic, stomach, and brain cancer receive two points and are automatically considered at high risk for VTE and thus were eligible for the AVERT trial. Other cancer types receive one or no points based on the Khorana score, and therefore required one or two additional thrombotic risk factors, respectively, to meet AVERT study inclusion criteria.
If a patient was eligible for the study by Khorana score, the next step required evaluation for AVERT exclusion criteria. Patients were excluded if they had “any condition that put them at an increased risk for bleeding.” This is an arguably subjective designation, and further details on what conditions were included in this category were not reported. Patients with a glomerular filtration rate (GFR) of less than 30 mL/min were excluded and few patients (2.9%) in this study had a GFR of 30 to 50 mL/min. Notably, antiplatelet therapy did not exclude patients from consideration — 22.8 percent of patients in the trial were receiving concomitant antiplatelet agents.
How does this study influence our clinical management of ambulatory patients with cancer? For clinical purposes, it makes sense to follow the AVERT algorithm (Figure) as a template for decision making regarding which patient to treat with apixaban 2.5 mg twice daily for VTE prophylaxis.
However, given the complexity of the selection criteria, the feasibility of identifying patients appropriate for thromboprophylaxis in real-world practice remains unclear. Furthermore, once thromboprophylaxis is initiated, patients may require additional monitoring given chemotherapy-associated risks of thrombocytopenia, renal insufficiency, and others that may increase bleeding risk. Potential system-based efforts to facilitate use of thromboprophylaxis could include the creation of tools in the electronic medical record to calculate the Khorana score and alert providers to potential treatment candidates or the development of designated anticoagulation clinics (as previously reported by Dr. Steven Ades and colleagues).4 The financial impact of apixaban must also be considered.
In Brief
In conclusion, the AVERT data support a change in our approach to thromboprophylaxis in ambulatory patients with cancer, as the trial indicates that apixaban 2.5 mg twice daily is effective and relatively safe. Three major VTE events are prevented at the cost of one major bleed, the majority of which are not clinical emergent bleeds. An additional study of thromboprophylaxis with rivaroxaban is ongoing and is expected to shed further light on best management of ambulatory cancer patients at risk for VTE.5 The feasibility of incorporating patient selection, monitoring, and cost into clinical practice is an important consideration that requires further investigation.
References
Competing Interests
Dr. Jori May indicated no relevant conflicts of interest. Dr. Moll has been a consultant for Janssen.