From Wan JCM, White JR, Diaz LA Jr. “Hey CIRI, What’s My Prognosis?” Cell. 2019;178:518-520. Used with permission.
From Wan JCM, White JR, Diaz LA Jr. “Hey CIRI, What’s My Prognosis?” Cell. 2019;178:518-520. Used with permission.
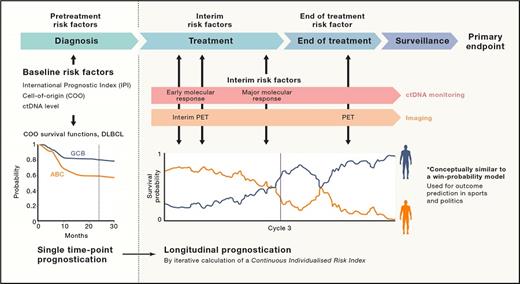
A new patient with diffuse large B-cell lymphoma (DLBCL) has an International Prognostic Index (IPI) of zero and is told that she has a 90 percent chance of staying in remission two years after completing R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy. A young patient with unmutated IgVH chronic lymphocytic leukemia (CLL) with normal cytogenetics is told that he has a 50 percent chance of maintaining a remission at five years following treatment with FCR (fludarabine, cyclophosphamide, and rituximab). For both patients however, these are just statistics, and they do not provide any insight into whether the patient in front of you will be in the 10 percent of patients with DLBCL who relapse, or whether the patient’s unmutated IgVH CLL will relapse within a year of completing FCR. In the era of alternative, non–chemotherapy-based effective therapies for these diseases, better individual predictors of outcome, rather than relying on the presence or absence of relapse over time, has the potential to spare a patient ineffective therapies and to direct them earlier to more effective therapies. This is the power in the Continuous Individual Risk Index (CIRI) developed and validated by Dr. David M. Kurtz and colleagues and published recently in Cell.
CIRI is a dynamic risk assessment that uses established pretreatment and interim-treatment risk factors for specific cancer subtypes to reassess the probabilities of an individual’s outcome over time. In DLBCL, these risk factors include the IPI, cell of origin of the tumor, interim positron emission tomography imaging assessment, pretreatment circulating tumor DNA burden, early molecular response, and major molecular response. In CLL, these risk factors include clinical and cytogenetic risk indices, peripheral blood minimal residual disease, and choice of therapy. Dynamic risk assessment in this system is modeled after “win-probability” models in which risk predictions are continuously assessed over time, considering additionally collected longitudinal data. It has the capacity to make outcome predictions at specific timepoints using a naïve Bayes approach, as well as to make longitudinal survival predictions using proportional hazard modeling and Bayesian analysis. In the validation cohorts for both DLBCL and CLL, CIRI closely calibrated to observed outcomes within ± 5 percent. In both models, CIRI outperformed any of the individual risk factors. Furthermore, because choice of therapy was considered a risk factor in the CIRI-CLL modeling, CIRI-CLL could be assessed for its power as a predictive biomarker for response to a specific therapy; it indeed could predict for benefit from FCR over alternative immune-chemotherapies following a period of induction therapy and an interim biomarker/response analysis. This model is applicable to other cancer subtypes as well; the authors present their modeling following neoadjuvant chemotherapy for breast cancer as an example.
In Brief
One of CIRI’s weaknesses is that inherent in this model is the initiation of established standard-of-care therapies for the disease in question. Our DLBCL or CLL patient ideally would want to know before even starting a therapy whether it will benefit them in a meaningful way. However, in two important ways it is a significant improvement over just waiting for a relapse. First, it may help to identify a group of patients at the end of standard-of-care induction therapy who are at high risk of relapse and therefore most likely to benefit from alternative consolidation or maintenance strategies. In DLBCL and CLL, consolidation or maintenance strategies have generally not proven beneficial for the population as a whole, but this lack of benefit may not hold true if we are able to identify the subgroup at highest risk of relapse. Second, if we can use these models at an early interim time point, before the end of induction therapy, to identify patients for whom this therapy is unlikely to produce lasting benefit, we can switch treatments to something more likely to be effective. For the patient with DLBCL, this may mean earlier treatment with chimeric antigen receptor T-cell therapy, and for the patient with CLL, this may mean moving on to any of the available targeted therapies or their combinations. Ideally, we are moving toward better pretreatment genomic, immunologic, and molecular prognostic modeling but we should recognize the progress and potential power a dynamic risk model such as CIRI offers.
Competing Interests
Dr. Jacobson indicated no relevant conflicts of interest.