Raymond (patient name has been changed for privacy) died on New Year’s Eve, only a few months after his initial diagnosis. He had achieved complete remission with standard chemotherapy, but the cytogenetics revealed an MLL-rearrangement and foretold of the adverse prognosis that patients and clinicians fear. He stoically endured three cycles of consolidation chemotherapy during workup for allogeneic bone marrow transplantation, but unfortunately relapsed two weeks after completion of chemotherapy and was refractory to all salvage options. Ray lived long enough to see his young son win his football grand final and to spend Christmas with his family, but by all other measures, nowhere near long enough.
Relapsed acute myeloid leukemia (AML) rapidly evolves into a chemotherapy-resistant disease and is incurable with standard approaches. The genetic factors driving the leukemia also govern chemotherapy resistance and prognosis after treatment.
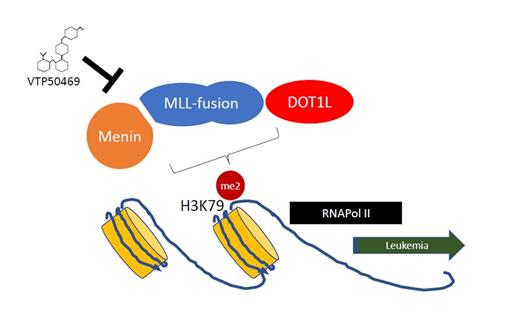
MLL-rearranged (MLL1, KMT2A) leukemias are an aggressive clinical subgroup of AML and acute lymphoblastic leukemia (ALL) with poor clinical outcomes, particularly in the context of therapy-associated AML and infant ALL. In the past 10 to 15 years, the molecular pathways governing MLL-rearranged oncogenic transformation have been carefully elucidated, demonstrating the importance of MLL-fusion protein interactions with chromatin-associated protein complexes. This work has led to the identification of novel drug targets, including DOT1 inhibitors,1 bromodomain inhibitors,2,3 and drugs that interfere with the Menin-MLL1 interaction.4 Menin binds to the N-terminus of MLL-fusion proteins and is required for MLL-fusion proteins to regulate aberrant gene expression pathways via the action of DOT1L.
Menin-MLL binding is required for the activation of MLL-fusion target genes via DOT1L regulation of H3K79me2 at specific loci. Interfering with this interaction may represent a new treatment for MLL-rearranged leukemia.
Menin-MLL binding is required for the activation of MLL-fusion target genes via DOT1L regulation of H3K79me2 at specific loci. Interfering with this interaction may represent a new treatment for MLL-rearranged leukemia.
In an exciting recent publication, Dr. Andrei Krivtsov and colleagues used an iterative structure–based design process to identify a highly active inhibitor of the Menin-MLL protein-protein interaction, VTP50469, with a favorable pharmacokinetic profile. The crystal structure confirmed binding within the Menin-MLL binding pocket, and validation studies showed that this compound had antileukemic activity evidenced by reduced cell growth, differentiation, and increased apoptosis. VTP50469 rapidly suppressed the gene expression program in MLL-rearranged cell lines and showed activity at low nM concentrations in various cellular contexts. Interestingly, the kinetics underlying the suppression of gene expression were more rapid than that seen with some other epigenetic therapies such as DOT1L inhibitors. Despite the remarkable efficacy, the effect of VTP50469 seemed to be limited to a discrete subset of MLL-fusion target genes including MEIS1, MEF2C, and JMJD1C, but not the HOXA cluster. This specificity may have been related to genes with the highest occupancy of Menin and DOT1L binding, though it remains unclear why some MLL-fusion target genes are insensitive to the effect of this inhibitor (Figure).
Importantly, this drug was orally bioavailable and showed efficacy in MLL-rearranged murine AML and patient derived xenograft samples from MLL-rearranged AML and ALL. The in vivo effects confirmed the cell culture findings with differentiation of leukemic blasts, on target suppression of aberrant gene expression, and on prolonged survival. Indeed, some recipient mice appeared to be cured, with long-term survival exceeding 450 days. This long-term disease-free survival is rarely seen with monotherapy, or even when combined with chemotherapy.5 Major dose-limiting toxicities were not observed in this study.
In Brief
These exciting preclinical results provide optimism but will need to be validated with prospective clinical studies. It is fair to say that there has been only modest efficacy of single-agent chromatin modifying agents such as DOT1L inhibitors6 and bromodomain inhibitors.7 It may be that the long-term role of such agents is in combination with chemotherapy, or to eliminate minimal residual disease after treatment. We have come a long way in the past few years with new drugs for leukemia including venetoclax combinations, monoclonal antibodies, and IDH1/2 or FLT3 inhibitors. The next challenge will be to convert these impressive response rates into long-term disease control or cure by incorporating these novel agents into our up-front therapeutic algorithms, following the example par excellence from acute promyelocytic leukemia. These discoveries may come too late for patients such as Raymond, but the reality for those working on the leukemia service is that the next patient is never far away. We are all inspired by the hope that lies on the horizon.
References
Competing Interests
Dr. Squires and Dr. Lane indicated no relevant conflicts of interest.