Unbeknownst to many hematologists and oncologists, a potentially earth-shattering revolution of man versus machine is occurring in pathology. Computers and even pigeons (!) are being trained to distinguish malignant from nonmalignant tissues based on digitized slide images.1-4 While there is a seemingly endless supply of pigeons, their diagnostic skills, like those of humans, require de novo training for each flock member; by contrast, the potential role of computer algorithms is facilitated by the ease and cost-effectiveness of propagation.
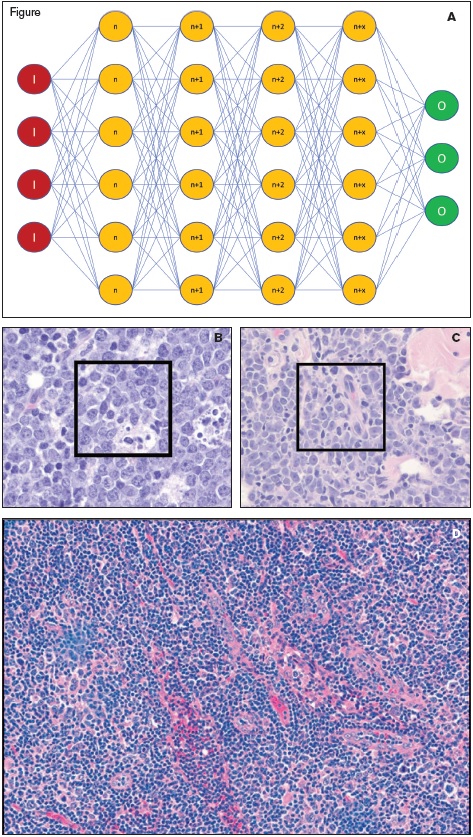
(A) Schematic of a convolutional neural network (CNN) with input (red circles marked with an “I”), multiple hidden layers of the network (orange circles representing layers n through n+x, where x is the number of layers and was 82 in the optimal CNN), and the output (green circles marked with an “O”). (B and C) Images of Burkitt lymphoma (B) and diffuse large B-cell lymphoma (C) with subcroppings used for input data for the training of the CNN indicated by the black box insets (reprinted with permission from the ASH Image Bank). (D) An example of an image of DLBCL that was diagnosed incorrectly as BL due to the large number of infiltrating small lymphocytes (reprinted with permission from Mohlman JS et al. Improving augmented human intelligence to distinguish Burkitt lymphoma from diffuse large B-cell lymphoma cases. Am J Clin Pathol, 2020; doi:10.1093/ajcp/aqaa001).
(A) Schematic of a convolutional neural network (CNN) with input (red circles marked with an “I”), multiple hidden layers of the network (orange circles representing layers n through n+x, where x is the number of layers and was 82 in the optimal CNN), and the output (green circles marked with an “O”). (B and C) Images of Burkitt lymphoma (B) and diffuse large B-cell lymphoma (C) with subcroppings used for input data for the training of the CNN indicated by the black box insets (reprinted with permission from the ASH Image Bank). (D) An example of an image of DLBCL that was diagnosed incorrectly as BL due to the large number of infiltrating small lymphocytes (reprinted with permission from Mohlman JS et al. Improving augmented human intelligence to distinguish Burkitt lymphoma from diffuse large B-cell lymphoma cases. Am J Clin Pathol, 2020; doi:10.1093/ajcp/aqaa001).
These algorithms are part of the umbrella term artificial intelligence (AI). In the case of pathology, AI can take numerous different forms, from counting mitotic figures or Ki67-stained cells to outright diagnosis of neoplasia and cancer subclassification. Diagnostic AI requires extensive training of the algorithm using large numbers of individual attributes (morphologic features) that can be fed into a multilayer computer process that recognizes diagnosis-specific features. This multilayer feature extraction and diagnostic recognition is termed a convolutional neural network (CNN; Figure 1A). While the early studies on CNNs in diagnostic pathology have focused on solid tumors, Dr. Jeffrey Mohlman and colleagues have illustrated the use of CNNs in the occasionally challenging, but clinically important, distinction between Burkitt lymphoma (BL) and diffuse large B-cell lymphoma, not otherwise specified (DLBCL, NOS).
BL is a highly aggressive malignancy comprised of monomorphic medium sized B cells with high mitotic index, numerous tingible body macrophages creating a “starry sky” pattern, and a canonical MYC translocation. However, there is no individual feature that is specific for BL, and the diagnosis rests on the incorporation of morphologic, immunophenotypic, and molecular/genetic findings.5 Luckily, BL has an excellent outcome if timely diagnosis and treatment takes place.6 This creates diagnostic pressure on the pathologist to quickly identify BL from morphologic mimics such as DLBCL, and high-grade B-cell lymphomas with MYC and BCL2 and/or BCL6 rearrangements (double-/triple-hit lymphomas) as these have different treatment regimens and prognosis.7 However, the need for molecular/genetic findings can delay the diagnosis of BL and may still not produce a definitive diagnosis.
Dr. Mohlman and colleagues therefore created a CNN that can identify morphologic features down to the pixel level. To create the CNN, diagnostic haematoxylin and eosin-stained slides of 34 cases of BL and 36 cases of DLBCL were digitally scanned at 200x magnification. Each digitized slide was then broken down into multiple JPEG files that were 1,712 × 1,112 pixels in size. This created a training set of 6,033 images of BL and 4,800 images of DLBCL. Parts B and C of the Figure illustrate the CNN input material, individual subcropped images (indicated by the inset black boxes) from larger tissue profiles of BL and DLBCL. While various subcrop sizes and layers of the network were explored, the optimal CNN used 672 × 672 pixels subcrops scaled down to 224 × 224 pixels and had a depth of 82 network layers. In total, 258,327 attributes were used to classify each individual image as either BL or DLBCL (including multiple different morphologic variants) in the training set.
To assess the accuracy of their CNNs, the researchers tested the CNN against a new cohort of nine cases of BL (1,637 total images) and 10 cases of DLBCL (730 total images). Their best performing algorithm had an accuracy of 87.6 percent for all images and correctly diagnosed 17 of 19 unknown cases overall. Of the 17 correct cases, 10 had 100 percent of images classified correctly, five cases had 76 to 99 percent of images classified correctly, and two had 50 to 75 percent classified correctly. Complicating factors that affected the success of the CNN included varying proportions of non-neoplastic tissue (including parenchymal tissue, fat infiltration, and non-neoplastic infiltrating lymphocytes), crush artifact, and dispersion/noncohesion of the neoplastic cells (such as fixation artifact; Figure 1D). These types of samples were underrepresented in the training set, and more replete training might improve the diagnostic precision of AI.
In Brief
These exciting findings highlight the diagnostic prowess of AI and raise the controversial question of whether it will eventually replace human microscopists. However, this hematologist audience should not worry about the potential loss of their friendly neighborhood pathology consultant. For all pathology AI development, a pathologist’s diagnosis is the gold standard, underscoring the importance of these professionals. As the understanding of pathobiology expands, pathologists develop and apply new classification schemes that require the training of new computational algorithms. Thus, “the reports of [the death of pathology] are greatly exaggerated.” In fact, computers will serve their pathology overlords. As pathologist workloads increase in number and complexity, AI can help prioritize cases, focus pathologist attention on areas of concern, integrate multiple data types, and provide objective data to help clarify borderline diagnoses in a timely fashion for the appropriate diagnosis and treatment of the patient.
References
Competing Interests
Dr. Brain and Dr. Kim indicated no relevant conflicts of interest.