On December 1, 2019, the World Health Organization was informed of cases of pneumonia with unknown etiology in Wuhan City, China. A novel beta coronavirus, Sars-CoV-2, was soon identified as the causative agent of the outbreak, termed COVID-19.1 By May 5, 2020, there were more than 3.5 million documented cases worldwide. The clinical course ranges from asymptomatic carrier to rapid and fatal respiratory failure. Initial reports revealed lymphopenia and dramatically elevated cytokine production, sometimes referred to as cytokine storm, in severely ill patients.2,3 Importantly, the degree of lymphopenia and cytokine production correlates with disease severity and mortality, suggesting that immune dysfunction may drive tissue pathology. Careful elucidation of the nature of both innate and adaptive immune dysfunction in patients with COVID-19 is therefore essential.
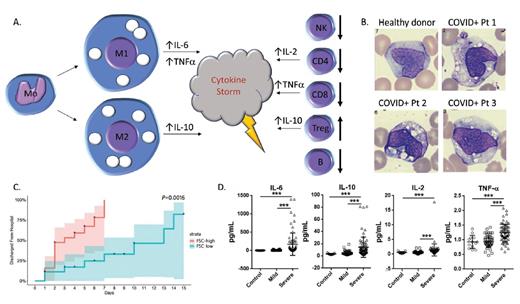
(A) Schematic of the abnormal production of circulating macrophages and monocytes as well as changes in T cell subsets in peripheral blood from patients with COVID-19. (B) Peripheral blood monocytes/macrophages from healthy donor (upper left) and three COVID-19-positive patients (images reprinted with permission from Zhang D et al. MedRxiv preprint; doi: 10.1101/2020.03.24.20042655). (C) Time to hospital discharge of patients with COVID-19 who were stratified by levels of forward scatter in monocytes (reprinted with permission from Zhang D et al). (D) Comparison of IL-6, IL-10, IL-2, and tumor necrosis factor a levels in healthy controls and COVID-19-positive patients with mild versus severe disease; ***, p<0.001 (reprinted with permission from Shi Y et al. MedRxiv preprint; doi: 10.1101/2020.03.12.20034736).
(A) Schematic of the abnormal production of circulating macrophages and monocytes as well as changes in T cell subsets in peripheral blood from patients with COVID-19. (B) Peripheral blood monocytes/macrophages from healthy donor (upper left) and three COVID-19-positive patients (images reprinted with permission from Zhang D et al. MedRxiv preprint; doi: 10.1101/2020.03.24.20042655). (C) Time to hospital discharge of patients with COVID-19 who were stratified by levels of forward scatter in monocytes (reprinted with permission from Zhang D et al). (D) Comparison of IL-6, IL-10, IL-2, and tumor necrosis factor a levels in healthy controls and COVID-19-positive patients with mild versus severe disease; ***, p<0.001 (reprinted with permission from Shi Y et al. MedRxiv preprint; doi: 10.1101/2020.03.12.20034736).
Evidence suggests that excessive macrophage activation may drive dysregulation of the innate response.4 Dr. Dan Zhang and colleagues demonstrated that macrophages and monocytes express ACE2, the surface receptor for SARS-CoV and SARS-CoV-2, in agreement with previous data.5 They then used flow cytometry to identify aberrant populations of monocytes and macrophages in COVID-19–positive patients. They found a relative decrease in classical monocytes (CD14 bright, CD16 dim/negative) in patients with COVID-19, and a concomitant increase in nonclassical monocytes (CD14 dim, CD16 bright). This skewing of monocyte populations was associated with the abnormal presence of macrophages with high forward scatter properties in the peripheral blood of patients with COVID-19 (Figure 1A). These macrophages were present in 28 (100%) of 28 COVID-positive individuals, but in none of the healthy controls (0/16 controls), or in those persons with other infections (HIV, tuberculosis, H1N1 influenza A, or Hantaan orthohantavirus). They represented a mix of the proinflammatory M1 subtype (expressing CD80 and typically associated with high IL-6 production) and the anti-inflammatory M2 subtype (expressing CD163 and CD206 and associated with IL-10 secretion). IL-6 in particular has been implicated as a key effector masterminding the cytokine storm found in patients with COVID-19. Peripheral blood smears from infected patients morphologically supported this finding, demonstrating increased numbers of macrophages with large, coalescent vacuoles (Figure 1B). Intriguingly, Dr. Zhang and colleagues showed the number of these atypical vacuolated monocyte/macrophages correlates inversely with time to discharge, indicating the potential prognostic value of the findings (Figure 1C).
Early reports on the adaptive response to SARS-CoV-2 indicated that the lymphopenia seen in COVID-19 patients is particularly pronounced among antigen experienced CD4+ and CD8+ T cells.6 Dr. Yaling Shi and colleagues also identified that lymphocyte numbers were substantially lower in severely ill patients, though circulating neutrophils were increased. The authors then assessed circulating lymphocyte populations by flow cytometry and identified significant reductions in CD4+ and CD8+ T cells in both mild and severe cases, with no difference between mild and severe. The authors also identified reductions in CD19+ B cells as well as CD16+ CD56+ CD3– natural killer cells, but not CD16+ CD56+ CD3+ natural killer/T cells. The authors further document an increase in CD4+ CD25+ CD127– T regulatory cells, which can be seen in the setting of uncontrolled inflammation (Figure 1A). In agreement with prior reports, the researchers showed increased tumor necrosis factor-α, IL-2, IL-6, and IL-10, with IL-6 and IL-10 particularly high in severely ill patients by flow cytometry and enzyme-linked immunosorbent assay.6 They did not see an alteration in interferon-γ production.
In Brief
These two studies illustrate the emerging data on abnormalities of the peripheral blood that can be seen in COVID-19–positive patients. These findings suggest that there is a shift of classical monocytes to more mature macrophages with increased proinflammatory function. There seems to be a relative increase in T regulatory cells, which may also be associated with dysregulated inflammation. However, while the broad phenotypic patterns of innate and adaptive responses have now been mapped, the specific functional subsets that compose these populations, and their contribution to viral clearance versus tissue pathology, remain unclear. Does the degree of vacuolization correlate with specific macrophage phenotypic or functional subsets, or is it a general property of effective responses? Even the origin of the monocytopenia and lymphopenia in the setting of a hyperinflammatory antiviral state is not clear. Evidence indicates SARS-CoV-2 induces a type III response dominated by Th17 cells despite the lymphopenia7 and the key mediator IL-6 typically results in skewing of CD4+ T cells away from T regulatory cells towards Th17 type cells – the opposite of what was found in these studies. Additionally, while other CD4+ and CD8+ T cells are reduced, their cytokine products are paradoxically increased. These findings may point to significant production of monocytes and T lymphocytes and their inflammatory cytokines with concomitant destructive, consumptive, or sequestrative processes, resulting in the particular cytopenias. Regardless, these distinct changes in the peripheral blood may be hallmarks of the dysregulated inflammatory state and may help stratify those patients who are likely to need more intensive care, as well as encourage investigations into adjuvant therapeutic options.
References
Competing Interests
Dr. DiToro and Dr. Kim indicated no relevant conflicts of interest.