Satisfactory control of TP53-mutated myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML) remains elusive, and even within poor risk groups of MDS with complex karyotypes, TP53 mutation status can identify patients with distinctly poorer outcomes.1
p53 protein is activated in response to cellular stress and subsequently activates cell cycle arrest, senescence, and/or apoptosis. Mutated p53 occurs in an array of malignancies, playing a critical role in disease evolution.2 Mutant p53 dysfunction facilitates chemoresistance, and hematopoietic clones defined by TP53 mutations are enriched after chemotherapy exposure and are frequently seen in therapy-related myeloid neoplasms, suggesting that they harbor and/or confer intrinsic resistance to genotoxic stress.1,3
The adverse prognostic implications of TP53 mutations are recognized throughout a wide range of malignancies, often independent of other risk factors (age and cytogenetics included). In the MDS setting, this holds true despite studied association with increased blast percentage, thrombocytopenia, and complex karyotypes.1,4–6 TP53-mutated AML forms a subset with poor response to standard cytotoxic chemotherapy (28-42% overall response rates) and shortened overall survival (OS), with median survival of four to nine months.1,4,5 These patients tend to be older, and almost all have karyotypes that are associated with unfavorable risk.4
Hypomethylating agents (HMAs) such as azacitidine (AZA) and decitabine have shown limited activity in patients with TP53-mutated AML and MDS, with complete remission (CR) rates of 15 to 20 percent and a median OS of around six months. Venetoclax (VEN), a selective small-molecule BCL2 inhibitor, has demonstrated promising data regarding responses in TP53-mutated AML when combined with hypomethylating agents, noting that duration of response remains short lived,7,8 and TP53 loss is identified in resistance to VEN and HMAs.9 These agents have added to our clinical algorithms; however, there is a clear need for novel therapies to overcome TP53-mediated resistance to VEN and HMAs in MDS and AML.
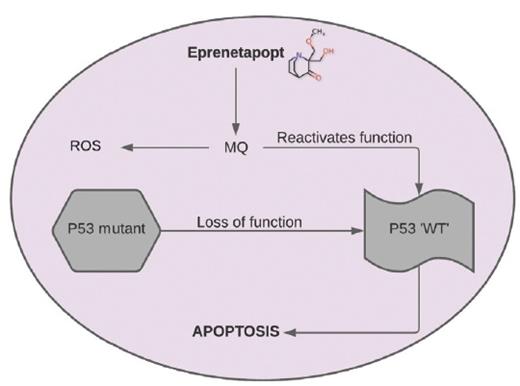
To try to improve these outcomes, the Groupe Francophone des Myélodysplasies, led by Dr. Thomas Cluzeau, used eprenetapopt (APR-246) in combination with AZA in patients with TP53-mutated MDS and AML. Eprenetapopt is a novel, first-in-class reactivator of mutant p53. The compound is converted to methylene quinuclidinone, which binds covalently to cysteines in mutant p53 or unfolded wild-type p53, thereby restoring its wild-type conformation (Figure).10 In doing so, cells acquire functions promoting apoptotic mechanisms and subsequent cell cycle arrest/senescence.
Eprenetapopt is converted to methylene quinuclidinone (MQ), a Michael acceptor that binds to mutant p53, restoring wild-type (WT) conformation and resulting in apoptosis. Eprenetapopt also generates reactive oxygen species (ROS) by disturbing cellular redox balance.
Eprenetapopt is converted to methylene quinuclidinone (MQ), a Michael acceptor that binds to mutant p53, restoring wild-type (WT) conformation and resulting in apoptosis. Eprenetapopt also generates reactive oxygen species (ROS) by disturbing cellular redox balance.
This phase II open-label single-arm multicenter study included 52 patients with TP53-mutated MDS or AML. Eprenetapopt was administered by IV over six hours on days 1 to 4 of each 28-day cycle. Patients had a median number of one TP53 mutations, 21 patients (40%) had biallelic mutations, and the median variant allele frequency (VAF) was 23 percent. Eighty percent of patients had adverse cytogenetics. The overall response rate (ORR) in the MDS cohort was 62 percent including a 47 percent CR, and in the AML group, the ORR was 33 percent including a 17 percent CR rate. Importantly, 73 percent of patients who responded achieved TP53 negativity on next-generation sequencing defined by a VAF less than 5 percent, though fewer patients achieved deep molecular remission of less than 0.1 percent VAF. At a median follow-up time of 9.7 months, median OS was 12.1 months for MDS and 10.4 months for AML, with a trend for shorter OS in AML with more than 30 percent blasts (median, 3 months) versus MDS (median, 12.1 months) and low-blast-count AML (median, 13.9 months). Moreover, CRs were not achieved in patients with AML and blasts greater than 30 percent, suggesting that this disease may need additional cytoreductive agents to achieve optimal effect. The adverse effect profile included neurologic events that resolved upon cessation of therapy, including ataxia, cognitive impairment, acute confusion, and dizziness. These events were increased in patients with renal impairment; however overall, eprenetapopt was well tolerated and safe.
In Brief
This article provides some optimism that TP53 mutations may help to select therapy with novel agents that specifically target the mutant gene.11 Eprenetapopt provides us with glimpses of hope for TP53-mutated myeloid disorders that continue to have inferior clinical outcomes. Although this trial hints at improved outcomes compared to historical controls, formal phase III randomized trials are underway to determine whether this is the case (NCT03745716). We await with bated breath the outcomes, so our patients may have some hope in the treatment of this important disease.
Competing Interests
Dr. Royle indicated no relevant conflicts of interest. Dr. Lane has received research funding from Bristol-Myers Squibb (BMS) for work regarding azacitidine.