The Case
A 76-year-old man was diagnosed with myelodysplastic syndrome with excess blasts-2 (MDS-EB2). Cytogenetics were normal; no molecular mutations were assayed. He declined referral for allogeneic stem-cell transplantation and began treatment with azacitidine. He achieved a complete remission (CR) after cycle 3, with normalization of blood counts and transfusion independence. Three and a half years after initiating hypomethylating agent treatment, he developed persistent cytopenias. A repeat bone marrow biopsy was performed and showed relapsed MDS with 12 percent blasts and morphologic evidence of trilineage dysplasia (Figure 1). Next-generation sequencing performed on the bone marrow aspirate revealed four tier 1 mutations involving the DEAD-box helicase 41 gene (DDX41; 2 variants), JAK2 (variant allele frequency [VAF] = 4.7%), and TET2 (VAF = 1.5%). The DDX41 mutations identified included DDX41 c.415_418dup, p. Asp140fs with a VAF of 44.4 percent and DDX41 c.1588G>A, p. Gly530Ser (VAF = 3.9%). Conventional cytogenetic analysis showed a normal male karyotype (46, XY).
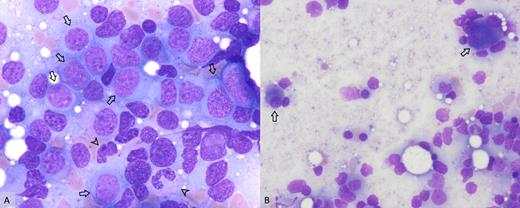
A) Increased myeloid blasts (arrows) and hypogranular neutrophils (arrowheads; 100×); B) dysplastic megakaryocytes including small, hypolobate forms and multinucleated forms (arrows; 50×).
A) Increased myeloid blasts (arrows) and hypogranular neutrophils (arrowheads; 100×); B) dysplastic megakaryocytes including small, hypolobate forms and multinucleated forms (arrows; 50×).
Question
What are the implications of DDX41 gene mutations in patients with MDS?
Explanation
The DDX41 gene is thought to be a tumor suppressor gene and is located on chromosome 5q35. Mutations involving this gene can result in defective splicing of pre-mRNA of oncogenes or other tumor suppressor genes and thus lead to the development of malignancy, with the most common being myeloid malignancies, including high-risk MDS, primary or secondary acute myeloid anemia (AML), and to a lesser extent, B-cell lymphomas and other solid tumors.
The DDX41 gene can undergo both germline and somatic mutations. Usually, the germline mutation acts as the “first hit” on one allele, while the somatic mutation acts as the “second hit” on the other. Germline alterations are predominantly out-of-frame insertions, loss of start codon, nonsense, or missense mutations that lead to complete loss of function. Somatic mutations are predominantly missense mutations. It is notable that germline mutations are not associated with somatic deletions of the DDX41 locus or frameshift mutations, as residual gene function is required for cell survival.
The frequency at which carriers of germline DDX41 mutations may develop a myeloid malignancy is still unknown. However, recent research suggests DDX41 mutations denote an increased risk of myeloid malignancy, which may not develop until the eighth decade, rather than following a Mendelian inheritance pattern. Many studies have shown that patients who harbor DDX41 mutations and develop myeloid malignancies are usually older and clinically present the same as patients with sporadic myeloid neoplasms. Blood counts of carrier individuals are usually normal; however, some may develop nonspecific cytopenias or monocytosis before eventually developing a myeloid neoplasm. Cytogenetic studies mostly show a normal karyotype; however, non-complex and complex karyotypes have been described, especially in patients who have mutations involving other genes such as TP53 or ASXL1 concurrently.
The frequency of DDX41 mutations among patients with MDS or AML is between 0.5 to 4 percent. The literature shows conflicting information regarding prognosis in these patients, with some studies showing poor prognosis and others favorable. Interestingly, it has been shown that these patients may respond to lenalidomide therapy.
It is worth mentioning that patients who receive a bone marrow transplant from a related donor may develop donor-derived leukemia; thus, patients and donors from the same family who have or are suspected to have myeloid neoplasms should be screened for DDX41 mutations.
Back to the Case
As the patient had two family members who died from leukemia (unknown types), and the presence of one DDX41 mutation with VAF approaching 50 percent, suspicion for an underlying germline DDX41 mutation was raised and germline testing was performed. The patient was found to be heterozygous for a pathogenic variant in DDX41. This alters the patient's diagnosis to myeloid neoplasm with germline DDX41 mutation. The patient and his family have consulted a geneticist.
Clinically, the patient joined a clinical trial of therapy with infusion cladribine and low-dose cytarabine and achieved a second CR.
Competing Interests
Dr. Al-Duwal and Dr. Cunningham indicated no relevant conflicts of interest.