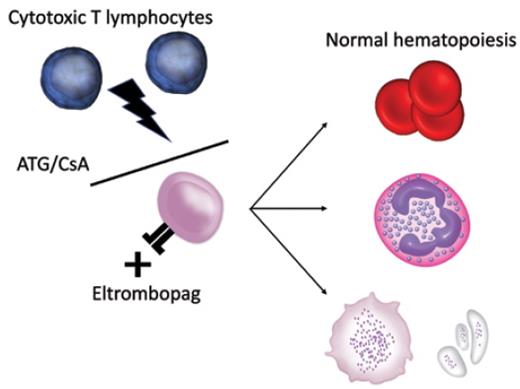
Acquired aplastic anemia (AA) is an immune-mediated bone marrow failure syndrome mediated by cytotoxic T lymphocytes targeting patients' own hematopoietic stem cells (HSCs).1 Although drugs, viral infections, hepatitis, and pregnancy can be a triggering event, in most cases the onset is idiopathic. Severity is classified by platelet, neutrophil, and reticulocyte count.2 The natural history of untreated severe AA (sAA) is one of rapid progression to complications of pancytopenia to eventual fatality. Since the use of anti-thymocyte globulin (ATG) was introduced for sAA in the 1970s, along with the addition of cyclosporin A (CsA) in the late 1980s, outcomes have significantly improved.2 However, hematologic complete response (CR) rates with combination immunosuppressive therapy (IST) remain low at 10 percent,3 and many patients relapse following initial response to IST.4 HSC transplantation (HSCT) remains the only curative option at this stage for sAA but is limited to patients younger than 40 years with human leukocyte antigen–matched donors, and comes with the risk of graft-versus-host disease (GVHD).
The 2017 phase I/II study from the National Heart, Lung, and Blood Institute examined the safety and efficacy of the thrombopoietin receptor agonist eltrombopag (EPAG) when added to ATG/CsA IST, using three different EPAG regimens in 92 consecutive patients with sAA.3 It was postulated that thrombopoietin signaling on HSCs could boost HSC survival and function. The results demonstrated a dramatic improvement in complete and overall response (OR) rate compared to historical controls and led to change in clinical management for patients who did not respond to ATG/CsA–based IST. However, important questions remained, including whether up-front EPAG/ATG/CsA was superior to ATG/CsA, and whether the addition of EPAG had consequences with regards to the evolution of secondary malignancy.
The landmark publication of the RACE trial in the New England Journal of Medicine in January 2022, is the first phase III, randomized control trial to examine the utility of EPAG in the upfront setting of the IST approach for sAA. The RACE trial was designed and directed by the European Society for Bone Marrow Transplantation Severe Aplastic Anaemia Working Party on the back of benefits derived by the addition of EPAG in the refractory setting in 2014.5,6
In this prospective, investigator-led open-label randomized controlled trial, involving 24 centers across six countries in Europe, 197 newly diagnosed patients with sAA who were ineligible for upfront HSCT were recruited onto the trial and randomized to either a standard of care (SoC; n=101) or experimental (n=96) group who received 150 mg oral EPAG daily from day 14 to six months, in addition to the standard ATG/CsA. Additional information on somatic mutations from bone marrow samples were collected using a 31-gene targeted molecular panel for an exploratory analysis to assess the frequency and variant allele frequency (VAF) of somatic myeloid cancer–associated mutations.
The study was powered to examine the primary end point of hematologic CR at three months, which improved from 10 percent with SoC to 22 percent in the experimental EPAG group (OR, 3.2; p=0.01). Multiple secondary end points were reported including OR, time to first response, best response, CR at six months, overall survival (OS), event-free survival (EFS), relapse rate (RR), clonal evolution, discontinuation of immunosuppression, and quality of life reported by the patient. Of these, key findings were a 27 percent increase in OR at six months with EPAG/ATG/CsA combination compared to SoC (68% vs. 41%, respectively) as well as more rapid time to first response (3 months vs. 8.8 months, respectively). No differences in two-year OS or RR were demonstrated; however, there was inferior EFS with SoC compared with EPAG/ATG/CsA (34% vs. 46%). Reassuringly, there were no significant toxicity concerns; 10 patients discontinued EPAG, primarily for liver function derangement, and two for increased bone marrow fibrosis, which was reversible.
Clonal hematologic evolution was a prespecified exploratory end point. At baseline, the frequency of somatic mutations, the mutated genes, and the median VAF were similar between the two treatment groups. Presence of baseline mutations was not significantly associated with OS or response. During the disease course, the frequency of mutations was surprisingly common and was increased from approximately 30 percent in both groups at baseline to 66 percent in ATG/CsA, versus 55 percent in the EPAG/ATG/CsA combination at six months, and further to 77 percent and 52 percent, respectively, at 24 months. Baseline or acquired mutations did not correlate with hematologic response or with OS, and these data elucidate only the emergence of clonal hematopoiesis rather than secondary malignancy, while noting that the median follow-up remains short at 24 months and that time to malignancy is usually five to 10 years postdiagnosis and treatment of sAA.
In Brief
In aggregate, the results of the RACE trial demonstrate the value of bringing EPAG into the frontline setting for IST in sAA, confirming the single arm studies previously demonstrated by Dr. Danielle M. Townsley and colleagues.3 Despite the impressive gains made in terms of CR and OR as well as time to first response with the addition of EPAG, the benefit on important overarching outcomes such as OS and RR is yet to be seen. Regardless, this important study identifies EPAG plus combination IST as the new SoC for patients with sAA who are ineligible for HSCT. Continued evaluation of the role of somatic mutations and clonal hematopoiesis in sAA may help identify those at high risk of progression to secondary myeloid malignancy and other complications of long-term growth factor and immunosuppressive therapy.
Combination treatment for severe aplastic anemia improves responses by preventing cytotoxic T-lymphocyte–mediated attack on hematopoietic stem cells and boosting stem cell function through thrombopoietin growth factor signaling. ATG/CsA, anti-thymocyte globulin/cyclosporine.
Combination treatment for severe aplastic anemia improves responses by preventing cytotoxic T-lymphocyte–mediated attack on hematopoietic stem cells and boosting stem cell function through thrombopoietin growth factor signaling. ATG/CsA, anti-thymocyte globulin/cyclosporine.
Competing Interests
Dr. Anderson and Dr. Lane indicated no relevant conflicts of interest.