Inherited bone marrow failure syndromes (IBMFSs) are hereditary disorders associated with progressive impairments in hematopoiesis, resulting in peripheral blood cytopenias and an inherent, but variable risk for myeloid neoplasms such as myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML).1 The etiologies for IBMFSs are diverse, with pathogenic or likely pathogenic germline variants in a variety of genes being implicated, affecting key cellular and biological pathways.2 Important examples of IBMFSs include telomere biology disorders (e.g., dyskeratosis congenita), disorders impacting ribosome biogenesis (Diamond-Blackfan anemia and Schwachman-Diamond syndrome [SDS]), and disorders resulting from defective DNA replication and repair (e.g., Fanconi anemia [FA]).
An important machine learning algorithm has recently been developed to help differentiate IBMFSs from acquired causes of bone marrow failure. It relies on predictors such as telomere lengths, abnormal cutaneous findings, macrocytosis, presence of physical abnormalities, pedigree charting, and the presence of a glycosylphosphatidylinositol–deficient/paroxysmal nocturnal hemoglobinuria (PNH)clone.3 In fact, it is extremely uncommon to find a PNH clone size greater than 1 percent in a patient with an IBMFS. Clonal hematopoiesis (CH) is defined by the acquisition of somatic pathogenic variants in hematopoietic stem and progenitor cells, with a potential to expand over time, based on cell intrinsic and extrinsic clonal selection pressures.4 When CH occurs in the context of leukemia-associated driver genes with a variant allele fraction of 2 percent or higher, in the absence of an underlying hematologic neoplasm it is referred to as a “CH of indeterminate potential” or age-related CH (ARCH).5 While somatic mosaic states in the hematopoietic compartment have been documented extensively as an age-related condition, context-specific mosaic states are rapidly being unraveled in patients with IBMFSs and germline predisposition syndromes (e.g., DDX41, GATA2, ETV6).5-7 Genes commonly mutated in ARCH include epigenetic regulators such as DNMT3A, TET2, and ASXL1, while splicing, signaling, and DNA damage response and repair pathway mutations are less frequent. Conversely, in therapy-selected CH, somatic mutations involving TP53 and PPM1D are frequent.8 While somatic mosaic states have been recognized as pathways toward progression to MDS/AML, the impact of individual variants, clonal selection pressures, clonal complexity, and the rate of progression, remain to be defined. Further heterogeneity is demonstrated by the fact that mosaic clones can also be associated with mosaic chromosomal abnormalities, which are not detected by conventional sequencing and cytogenetic studies and are associated with neoplastic transformation.8
Fascinating insights have been obtained in IBMFSs where dysfunctional hematopoiesis can be partially or fully compensated by somatic variants in the hematopoietic stem and progenitor cells, overcoming the fitness constraints that were imposed by the germline variants.9 While these escape mechanisms have adaptive origins, they can result in maladaptive consequences culminating in myeloid neoplastic transformation (MDS/AML).9 These somatic variants can be divided broadly into two categories:
Somatic variants that alleviate baseline fitness constraints by negating the underlying germline deficit through direct reversion or indirect compensation. These variants are unlikely to result in myeloid neoplastic transformation.
Somatic variants that attempt to relieve the germline competitive disadvantages by impairing cellular elimination. These variants have an increased risk for myeloid neoplastic transformation, since they impair critical tumor suppressor pathways.
This article provides a review of individual IBMFS categories and discusses their somatic landscapes and contributions toward progressive bone marrow failure and myeloid transformation.
SDS
This is an autosomal recessive disorder caused by biallelic germline mutations in the SBDS gene (usually compound heterozygous).10 The SBDS protein is required for the development of a translationally active 80S ribosomal subunit, which is created when SBDS cooperates with the GTPase ELF1 to catalyze the removal of EIF6 (eukaryotic translation initiation factor 6) from the 60S ribosomal subunit. In the absence of SBDS, EIF6 remains bound to the 60S subunit and inhibits it in joining the 40S subunit. In SDS cells, SBDS deficiency impairs the release of EIF6 from the nascent 60S subunit resulting in decreased ribosome translational efficiency and the activation of cellular senescence pathways.10 Affected patients with SDS have a very high risk for MDS/AML, often demonstrating somatic TP53 mutations as well as isochromosome 7q and deletion 20q. TP53 tumor suppressor pathways are activated by defective ribosome biogenesis and aberrant protein translation. In a seminal study, investigators demonstrated multiple independent hematopoietic clones occurring early in the life spans of patients with SDS, harboring heterozygous mutations involving TP53 or EIF6. They demonstrate that SBDS deficiency establishes a fitness constraint, driving the selection of somatic clones with two different outcomes. Somatic mutations involving EIF6 inactivate CIM6, ameliorating the underlying SDS ribosomal defect, enhancing clonal fitness, and improving bone marrow failure outcomes. Conversely, somatic TP53 mutations enhanced the leukemic potential by inactivating tumor suppressor checkpoints, without correcting the ribosomal defect. Biallelic TP53 alterations and TP53 multihit states (including structural and numerical abnormalities and copy neutral loss of heterozygosity) were associated with a high prevalence of AML.10
Telomere Biology Disorders
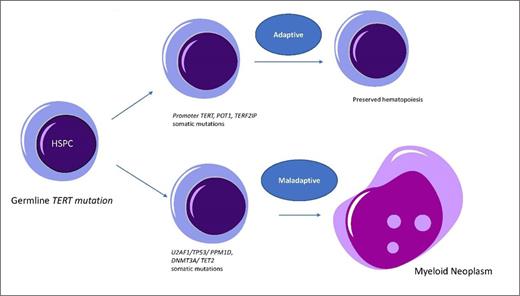
These are IBMFSs characterized by multiorgan involvement, often resulting in bone marrow failure, interstitial lung disease, nodular regenerative hyperplasia/ hepatic fibrosis, and an increased risk for MDS/AML. The diagnosis is established by demonstrating low mean telomere lengths in granulocytes and lymphocytes using a peripheral blood flow-fish (fluorescence in situ hybridization) assay.11 Germline mutations in the telomere apparatus can be identified in approximately 50 percent of patients (e.g., TERT, TERC, RTEL1, POT1, PARN, and TINF2). Somatic mosaic states have been detected in patients with telomere biology disorders with prevalence rates being higher than expected for ARCH. In a seminal study, approximately 30 percent of patients had somatic mutations involving the TERT promoter (19%), POT1, or TERF2IP (11%; Figure). The POT1 variants seen were similar to those seen in patients with familial melanoma and pancreatic cancer, where they have been associated with increased telomere lengths, indicative of complex compensatory mechanisms.12 In patients with germline defects in TERC (RNA template for the reverse transcriptase), somatic variants were identified in nuclear RNA exosome genes such as RBM7, SKIV2L2, and DIS3.12 Loss-of-function variants in these genes are known to increase upregulation of TERC levels. These variants were considered adaptive in nature, giving rise to somatic genomic reversion, and were much more prevalent in patients with telomere biology disorders who were MDS/ AML free (relative risk, 4.4; 95% CI, 1.2-16.7). On the other hand, somatic mutations involving genes such as U2AF1, PPM1D, TP53, DNMT3A, TET2, and signal pathway abnormalities have also been detected.13,14 While TP53 and PPM1D are central effectors of the DNA damage response evoked by telomere dysregulation, reasons behind the higher-than-expected frequency of U2AF1 mutations (spliceosome component protein) remain to be elucidated. While these variants temporarily stabilize bone marrow failure and increase bone marrow cellularity, they are associated with a higher risk of progression to MDS/AML.
FA
FA is an IBMFS that occurs secondary to defects in DNA damage response and repair, primarily affecting homologous recombination. Somatic mosaic states have been identified in patients with FA, with mild cytopenias and improving blood counts occurring because of the same.15 These patients are still at increased risk for clonal cytogenetic changes and somatic mutations in non-revertant cells that can result in myeloid neoplastic transformation. While in compound heterozygous FA–mutant patients’ somatic back mutations have been documented, in patients with homozygous defects, clonal populations of circulating lymphocytes with somatic missense and frameshift mutations in cis with the germline allele have been documented. These changes have been shown to repair the DNA damage response defect created by the germline state. In a seminal publication, mechanisms of clonal selective advantages in FA were recently described. More than 50 percent of patients with FA develop trisomy 1q (+1q), resulting in an extra copy of MDM4 (mouse double minute 4), which is able to decrease the transcription of TP53, leading to clonal selective advantages to the FA hematopoietic stem and progenitor cells.16 Additional leukemogenic clonal events noted included 3q+ involving the EVI1 gene, 7q-, and RUNX1 somatic mutations.16
As the field of clonal hematopoiesis testing evolves, adaptive and maladaptive somatic mosaic states in IBMFSs and germline predisposition syndromes (GATA2, DDX41, ETV6, ANKRD26, CEBPA, RUNX1, SAMD9/SAMD9L, etc.) continue to unravel, providing the scientific community with useful insight into disease biology and function. Therapeutic opportunities, including the role of gene editing in these conditions, can also be studied with greater clarity. At Mayo Clinic, we have a dedicated clinical service line assessing adaptive and maladaptive somatic mosaic states in IBMFS and germline predisposition syndromes (clinicaltrials.gov/ct2/show/NCT02958462). Important questions that remain to be answered include the clinical consequences of detecting somatic mosaic states in affected patients. While maladaptive somatic acquisitions can lead to myeloid neoplastic transformation, variability in progression and availability of less-than-optimal interventional strategies make treatment approaches, such as chemotherapy or stem cell transplantation, very challenging.
Competing Interests
Dr. Patnaik indicated no relevant conflicts of interest.