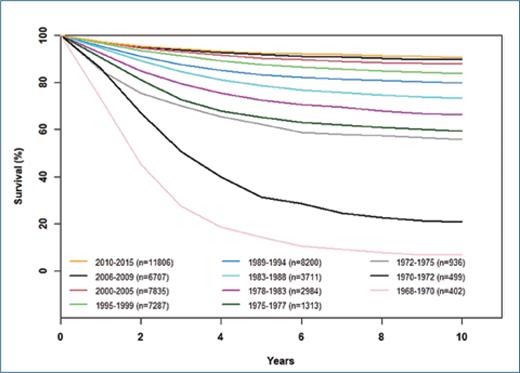
Multi-drug frontline chemotherapy regimens, tested and refined via pediatric oncology cooperative group clinical trials over the past five decades, have increased survival rates among children and adolescents/young adults (AYAs) with B-acute lymphoblastic leukemia (B-ALL), which now exceed 90% (Figure).1,2 In North America, current curative regimens comprise more intensive induction and post-induction phases lasting approximately nine months and a less intensive, but nonetheless essential, maintenance phase lasting approximately 18 months. Clinical and laboratory characteristics such as age and white blood cell count at diagnosis, involvement of the central nervous system and other extramedullary sites, leukemia-associated genetic alterations, and responses to initial chemotherapy based upon assessments of minimal/measurable residual disease have been used to support risk-adapted intensification (and de-intensification) of therapy when appropriate.3-6
Overall survival of children with ALL treated on legacy and contemporary Children’s Oncology Group clinical trials. Data are courtesy of the COG ALL committee and adapted from Raetz et al. Pediatric Blood & Cancer, 2023.15
Overall survival of children with ALL treated on legacy and contemporary Children’s Oncology Group clinical trials. Data are courtesy of the COG ALL committee and adapted from Raetz et al. Pediatric Blood & Cancer, 2023.15
Essential components of maintenance therapy include daily oral 6-mercaptopurine (~75 mg/m2) and weekly oral methotrexate (~20 mg/m2), with monthly doses titrated based upon hematologic and hepatic laboratory parameters. Recent studies by the Children’s Oncology Group (COG) have demonstrated that adherence to >95% of prescribed 6-mercaptopurine therapy is critical for minimizing the risk of relapse in pediatric and AYA patients with newly diagnosed B-ALL.7,8 Additional studies have shown that 6-mercaptopurine adherence and relapse risk may also be differentially influenced by age, race/ethnicity, socioeconomic status, and other demographic factors.9,10
In legacy frontline studies conducted during the 1980s, inclusion of intravenous vincristine and oral dexamethasone or prednisone “pulses” during 6-mercaptopurine/methotrexate-based maintenance therapy appreciably improved event-free survival (EFS) rates among pediatric patients with ALL.11 However, more recent trials focused on risk-adapted intensifcation of treatment following the induction phase have not detected a benefit of maintenance pulses in most patients with ALL (except those with IKZF1 deletions).12,13 As such, most modern ALL protocols in Europe do not include vincristine or steroid chemotherapy during the maintenance phase. In the recent COG AALL0932 phase III clinical trial, children with standard-risk B-ALL were randomized to receive vincristine/dexamethasone pulses every one versus three months during maintenance, which ultimately yielded no detectable differences in EFS.14 Results from AALL0932 have subsequently informed new treatment paradigms in North America with utilization of reduced-frequency pulses.
In their recent meta-analysis, Dr. Louise Guolla and colleagues extracted data from 25 studies published between 1965 and 2022 to assess the potential impact of vincristine/steroid maintenance pulses on EFS in pediatric patients (≤18 years old) with newly diagnosed B-ALL. Based on data from 12,513 patients, the authors ascertained that the clear benefit of maintenance pulses in historic trials (i.e., those conducted prior to the 1990s) was mitigated in more recent studies that employed a more intensive strategy for risk-adapted post-induction chemotherapy dosing prior to maintenance. Focused analysis of data from contemporary trials specifically demonstrated no deleterious impact of reduced-frequency vincristine/steroid pulses (or no pulses) on EFS, relapse risk, or overall surivival. Importantly, the inclusion of greater-frequency vincristine/steroid pulses was associated with increased toxicity, even in the historically less intensive maintenance phase of treatment, when patients and families often experience a more pronounced return to “normal life.” Adverse events assessed in the recent analysis included cytopenia, infection, osteonecrosis, and peripheral neuropathy; however, trends toward differences in toxicity were not always statistically ascertainable due to bias and/or wide confidence intervals given the heterogeneous datasets.
In Brief
This large-scale meta-analysis focused upon the potential impact of maintenance vincristine/steroid pulses on relapse risk, survival, and therapy-associated adverse events in pediatric patients with B-ALL highlights the importance of clinical trial investigation and the success of modern risk-adapted therapies developed by international pediatric oncology consortia. These results will undoubtedly inform the design of future clinical trials, with a critical overarching goal of successful cure at less cost for pediatric and AYA patients with ALL.
Competing Interests
Dr. Tasian indicated no relevant conflicts of interest.