Pathophysiology
Hemophilia is an X-linked disorder that is almost exclusively encountered in men. Both the factor VIII and IX genes are located on the X chromosome, and the deficiency states are known as hemophilia A and hemophilia B, respectively. Worldwide, hemophilia A occurs in 1 in 4,000 to 1 in 5,000 male births, while hemophilia B occurs in 1 in 15,000 to 1 in 30,000 male births.1 The enzymatic complex comprising the activated forms of factors VIII and IX is known as the “intrinsic tenase complex,” one of two in vivo pathways that activate substrate factor X. While factor VIIIa (FVIIIa) possesses no enzymatic activity, it is a required cofactor for FIXa in this complex, which also requires the presence of negatively charged phospholipids supplied by activated platelet membranes in the hemostatic plug. Hemophilia is classified according to the baseline level of FVIII or FIX, with clotting factor levels of less than 1%, 1-5%, and 5-40% reflecting “severe,” “moderate,” and “mild” cases, respectively.2
Since the implementation of safety measures to prevent the transmission of HIV and hepatitis C during plasma-derived clotting factor therapy in the late 1980s, the development of antibodies (i.e., “inhibitors”) that neutralize FVIII or FIX is now viewed as the most clinically significant complication of factor replacement therapies. The cumulative incidence of this phenomenon is approximately 30% among patients with severe hemophilia A compared with 2 to 4% in those with severe hemophilia B. Persistently high levels of these inhibitors negate the ability to control bleeding with conventional factor replacement therapies. While many can be eliminated via immune tolerance induction therapy, inhibitors persist in 5 to 7% of patients,3 who experience worse clinical outcomes and require alternate hemostatic therapies.
Clinical Presentation
The age and mode of clinical presentation, which is clinically indistinguishable between hemophilia A and hemophilia B, depend on the severity of the respective factor deficiency. Ideally, hemophilia is diagnosed before or shortly after birth when the mother is known to carry a pathogenic mutation; however, approximately one-third of new cases are classified as sporadic. Some neonates with previously unsuspected disease present with early birth-induced bleeding (with intracranial hemorrhage being the most feared complication) or abnormal bleeding following traumatic interventions such as circumcision. Thereafter, boys with severe hemophilia generally experience the hallmark joint and muscle bleeds when they begin to walk at approximately 12 months of age. In the natural course of untreated severe hemophilia, these “spontaneous” joint bleeds persist throughout life, generally with a frequency of two or more instances per month, leading to cumulative joint injury, end-stage arthritis, and crippling orthopedic disease by the third decade of life. Patients with non-severe hemophilia may experience fewer spontaneous bleeds that appear to resolve only when the endogenous FVIII/IX level is greater than 10 to 12%.4 However, it is not unusual for patients with previously undiagnosed, milder forms of hemophilia to present with bleeding after trauma or surgery in later childhood or even adulthood.
Diagnosis
Once clinically suspected, the diagnosis of hemophilia is straightforward. History of bleeding, extended X-linked family history, and assays of FVIII or FIX activity will generally reveal the diagnosis. A high index of suspicion for inhibitors necessitates vigilant screening throughout life, particularly after heavy exposure to factor replacement therapy.
Treatment
Following the initial success of plasma-derived concentrate therapies in the 1960s,5 self-administered, “on-demand” factor concentrates were widely adopted among the hemophilia community. However, the subsequent realization of the potential for HIV and hepatitis C transmission in donor plasma in the 1980s accelerated the drive to clone the genes for FVIII and FIX and produce recombinant FVIII and FIX concentrates. Thereafter, biotechnology researchers focused on developing extended half-life (EHL) clotting factors via PEGylation or the linkage of albumin or the Fc portion of immunoglobulin. The standard of care also shifted from “on-demand” therapy to continuous prophylaxis, with the goal of eliminating spontaneous bleeds completely. Furthermore, within the past decade, there has been an explosion in the development of newer, non-traditional hemostatic products. In 2017, an “FVIII mimetic” bispecific antibody (Emicizumab) was licensed for prophylaxis in patients with severe hemophilia A with or without inhibitors. Within the past year, adeno-associated virus (AAV)-mediated gene therapy has been approved for both hemophilia A and B. This milestone was achieved as a result of 30 years of basic and pre-clinical research in academic laboratories. The pipeline of agents in development remains extraordinarily robust and includes newer-generation approaches to gene therapy and/or repair, as well as an entirely new field focused on “re-balancing” therapies.6 These include siRNA or antibodies to knock down or inhibit natural anti-thrombotic pathways, including antithrombin, tissue factor pathway inhibitor, or the protein C/protein S pathway. Notably, these agents can be used to prevent bleeding in those with hemophilia A or B even when inhibitors are present.
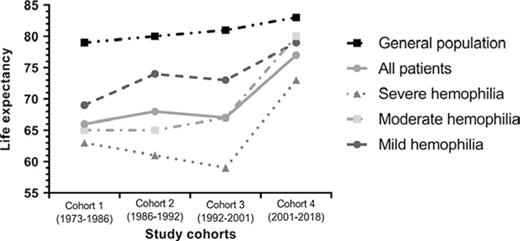
Future Directions
Prior to the emergence of clotting factor concentrates in the 1960s, patients with hemophilia had an average life expectancy under 20 years of age. After 1970, life expectancy among the hemophilia population in developed nations initially exhibited a significant improvement following the introduction of the above-mentioned therapies and the Comprehensive Care model. However, as exemplified by the Dutch national experience, a downturn occurred following the HIV era, lasting until approximately 2000, since when life expectancy has improved but remains below that of the general Dutch male population (Figure).7 However, this belies the fact that even in the more recent era, bleeding accounts for a significant proportion of deaths (25-30%) in patients with hemophilia, with rates of intracranial hemorrhage that are 3- to 13-fold higher than those in the general male population.7,8 Furthermore, health-related quality of life remains suboptimal in the hemophilia population, with joint disease being the primary determinant of this outcome, particularly among older adults.9 Preventing joint disease to improve quality of life and maximize patient participation in normal physical activities, school, and the workplace therefore remains a significant priority. Unfortunately, the high cost of these therapies has prevented their widespread adoption in lower-income countries,10 where the burden of disease remains high.
Median life expectancy in patients with hemophilia in the Netherlands between 1973 and 2018, compared with that in the general male population over time.
Hassan S, Monahan RC, Mauser-Bunschoten EP, et al. Mortality, life expectancy, and causes of death of persons with hemophilia in the Netherlands 2001-2018. J Thromb Haemost. 2021;19(3):645-653.
Copyright © 2020 The Authors. Journal of Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis
This is an open access article under the terms of the http://creativecommons.org/licenses/by-nc/4.0/ License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
Median life expectancy in patients with hemophilia in the Netherlands between 1973 and 2018, compared with that in the general male population over time.
Hassan S, Monahan RC, Mauser-Bunschoten EP, et al. Mortality, life expectancy, and causes of death of persons with hemophilia in the Netherlands 2001-2018. J Thromb Haemost. 2021;19(3):645-653.
Copyright © 2020 The Authors. Journal of Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis
This is an open access article under the terms of the http://creativecommons.org/licenses/by-nc/4.0/ License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
Conclusion
Despite the history of adversity in the treatment landscape for hemophilia, first based on the inability to prevent or treat bleeding and thereafter based on the tragic history of bloodborne viral transmission beginning in the 1980s, the future now seems bright. It remains to be seen how gene therapy — the traditional “mecca” of hemophilia treatment11 — will be adopted given concerns related to durability, possible adverse long-term outcomes,12 and the fact that protein therapies have certainly raised the therapeutic bar.
Competing Interests
Dr. Key has served as a consultant to Biomarin and Uniqure/CSL Behring and as Chair of the “Access to Insight” grants program sponsored by Novo Nordisk.