STUDY TITLE: Testing Early Treatment for Patients With High-Risk Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Leukemia (SLL), EVOLVE CLL/SLL Study
CLINICALTRIALS.GOV IDENTIFIER: NCT04269902
PARTICIPATING CENTERS: All Southwest Oncology Group sites (lead organization) and National Clinical Trials Network organizations, including the Alliance for Clinical Trials in Oncology, the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network Cancer Research Group, and the Canadian Cancer Trials Group
SPONSOR: National Cancer Institute
ACCRUAL GOAL: A total of 247 patients between two cohorts
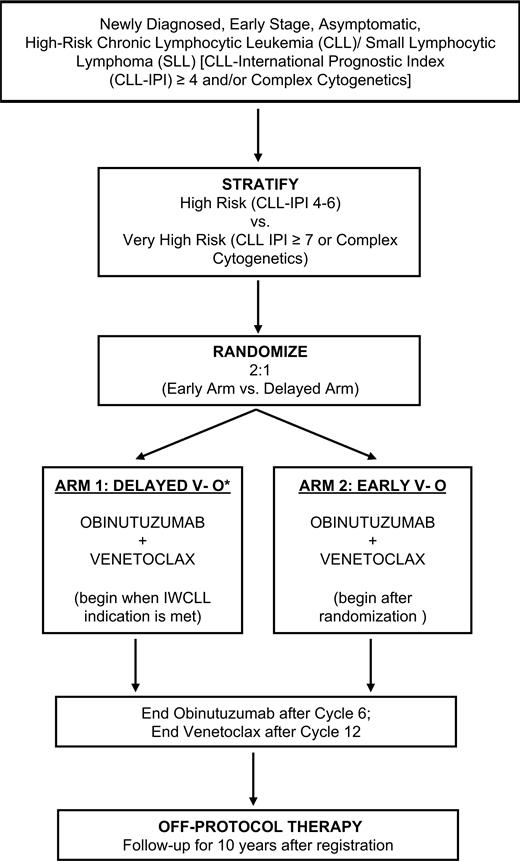
STUDY DESIGN: The EVOLVE CLL study is a phase III open-label, multicenter clinical trial designed to compare the outcomes of patients with high-risk chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) treated with immediate therapy consisting of 12 months of venetoclax and obinutuzumab (early VO) compared with those initially managed with active surveillance followed by 12 months of VO therapy when a traditional treatment indication develops (delayed VO). Adult patients with CLL/SLL diagnosed within 18 months of enrollment are eligible, provided they lack an indication for treatment by standard International Workshop on CLL criteria and have high-risk features as defined by a CLL International Prognostic Index (CLL-IPI) score of 4 or more and/or complex cytogenetics (≥3 karyotypic abnormalities) (Table).1,2 Participants are randomized two to one in favor of early VO. The primary endpoint is to evaluate whether early VO extends overall survival (OS) compared with delayed VO. There are multiple additional secondary endpoints, which will include a comparison between arms of response rates, progression-free survival (PFS), safety, time to second CLL-directed treatment, and rates of Richter transformation. Patient-reported outcomes will be used to compare participants in relation to health-related quality of life as determined per the Functional Assessment of Cancer Therapy–Leukemia scale. Several translational studies focused on the association of outcomes with minimal residual disease (MRD) status and baseline molecular features of disease are planned as integrated biomarkers.
CLL International Prognostic Index scores
| Characteristic . | Points . |
|---|---|
| Deletion 17p and/or TP53 mutation | 4 |
| Serum b2 microglobulin ≥3.5 mg/L | 2 |
| Unmutated IGVH gene status | 2 |
| Rai stage I-IV | 1 |
| Age >65 years | 1 |
| Characteristic . | Points . |
|---|---|
| Deletion 17p and/or TP53 mutation | 4 |
| Serum b2 microglobulin ≥3.5 mg/L | 2 |
| Unmutated IGVH gene status | 2 |
| Rai stage I-IV | 1 |
| Age >65 years | 1 |
Abbreviation: IGVH, immunoglobulin variable heavy-chain.
RATIONALE: The standard approach to all patients with CLL/SLL who lack an indication for treatment remains active surveillance (referred to as “watch and wait”), regardless of risk factors. However, this results in significant anxiety for patients and may allow for adverse clonal evolution, particularly for patients with unfavorable biologic features. Prior attempts to study early intervention with nonspecific chemotherapeutic agents in participants with asymptomatic CLL did not improve OS compared to delaying treatment, but there has been a remarkable shift over the past decade in the management of frontline treatment of CLL/SLL from chemoimmunotherapy to targeted therapy.3 These approaches include time-limited treatment with VO, as well as indefinite treatment with oral Bruton tyrosine kinase inhibitors, including ibrutinib, acalabrutinib, and zanubrutinib.4-7 These treatments are particularly effective in patients with CLL who are at the greatest risk of responding poorly to traditional therapy, such as those with a CLL-IPI score of 4 or more, or those with complex karyotype. Therefore, there is significant interest and rationale to study early intervention with targeted therapy in the high-risk CLL population. VO was selected for the early intervention regimen, as it is time-limited and more than 70% of patients achieve undetectable MRD status at the conclusion of treatment.8 It is also likely that early treatment for CLL, when the disease burden is lower, will reduce the risk of treatment-related tumor lysis syndrome. Overall, the hypothesis of the study is that early VO can improve survival of participants with newly diagnosed, asymptomatic, high-risk CLL when compared with delayed VO, as shown in the accompanying schema (Figure).
Treatment schema of EVOLVE CLL trial
*Treatment to be initiated once 2018 International Workshop on Chronic Lymphocytic Leukemia (iwCLL) indication is met.
Treatment schema of EVOLVE CLL trial
*Treatment to be initiated once 2018 International Workshop on Chronic Lymphocytic Leukemia (iwCLL) indication is met.
COMMENT: Clinical trials designed specifically for patients with CLL/SLL without a traditional indication for treatment are rare but needed to determine if active surveillance is still an appropriate strategy in the modern era of improved molecular risk stratification and highly effective targeted therapy. Although immediate treatment with ibrutinib in asymptomatic CLL/SLL improves PFS relative to no treatment, ibrutinib is associated with toxicities, and it is not clear that earlier treatment improves survival.9 Because a significant proportion of patients with CLL/SLL can be safely monitored for years and effectively managed when an indication for treatment arises, early prevention must be shown to improve survival to become practice-changing, as well as warrant its cost and potential toxicity. Will early VO improve survival of patients with high-risk CLL/SLL? Once fully accrued, approximately four years of follow-up will be needed for sufficient events to occur to compare the two approaches. Until that time, the CLL community will just have to watch and wait.
Disclosure Statement
Dr. Hill is a co-chair of the EVOLVE CLL study and has received research funding and consulting fees from Abbvie, Genentech, Pharmacyclics, AstraZeneca, and BeiGeine.