Vitamin A plays an important role in maintaining intestinal homeostasis1 and immune modulatory functions, including inducing tolerogenic tissue-resident macrophages.2,3 High-dose oral vitamin A is recommended by the World Health Organization (WHO) in children with measles at doses of 200,000 IU orally daily for two days to help improve mucosal immunity and clinical outcomes.4 Pooja Khandelwal, MD, and colleagues previously showed that children undergoing hematopoietic stem cell transplantation (HSCT) who had blood vitamin A levels below the median experienced a higher incidence of acute gastrointestinal (GI) graft-versus-host disease (GVHD).5 This observation is particularly relevant because acute GVHD is the most significant complication following allogeneic HSCT,6 and gut involvement is associated with increased mortality.7
With this background, Dr. Khandelwal and her research team hypothesized that oral vitamin A supplementation would reduce the incidence of acute gut GVHD by inducing tolerogenic tissue-resident macrophages2,3 and modulating T-cell trafficking to the gut by influencing the expression of the vitamin A-dependent chemokine CCR9.8 A randomized, double-blinded, placebo-controlled phase II clinical trial was performed at Cincinnati Children’s Hospital Medical Center comparing the use of vitamin A supplementation versus placebo prior to allogeneic HSCT. The dose of oral vitamin A was informed by the WHO recommendations on measles4 and confirmed in an independently performed safety study. A single 4,000 IU/kg (maximum 250,000 IU/kg) dose of vitamin A was given orally anytime within one week before initiation of conditioning chemotherapy. Canola oil served as a placebo. The primary study endpoint was the incidence of acute GVHD by day 100 after HSCT.
Eighty patients enrolled in the study, with 40 randomized to each arm. The median ages were 6.9 (range, 2-20) in the vitamin A arm and 8.5 (range, 1.1-28) in the placebo arm. Transplant demographics were balanced between both arms. Vitamin D sufficiency was ensured for all study subjects, as vitamin A and D act synergistically on similar nuclear receptors,9 making this an important variable to control.
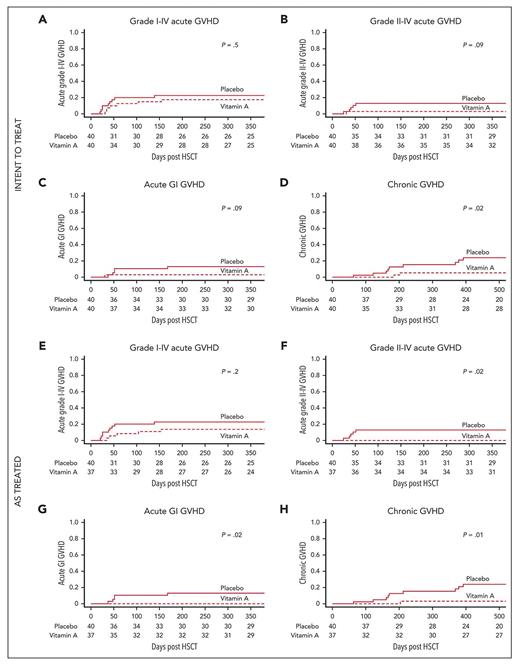
An intent-to treat analysis showed that the cumulative incidence of acute GI GVHD by day 180 post-HSCT was 2.5% in the vitamin A arm and 12.5% in the placebo group (p = 0.09; Figure). Surprisingly, the cumulative incidence of chronic GVHD was 5% in the vitamin A arm and 15.3% in the placebo group at one year post-HSCT (p=0.02). An “as-treated” analysis was also performed to further assess the biological effect of the intervention. The cumulative incidence of acute GI GVHD by day 180 post-HSCT was 0% in the vitamin A arm and 12.5% in the placebo group (p=0.02; Figure), while the cumulative incidence of chronic GVHD at one year post-HSCT was 2.7% in the vitamin A arm and 15.3% in the placebo group (p=0.01).
Recipients of vitamin A have lower cumulative incidence of graft-versus-host disease compared to placebo
Intent-to-treat analyses: cumulative incidence of (A) grade 1 to 4 acute GVHD (primary endpoint); (B) grade 2 to 4 acute GVHD; (C) acute GI GVHD; and (D) chronic GVHD (panels B-D: secondary endpoints). As-treated analyses: cumulative incidence of (E) grade 1 to 4 acute GVHD (primary endpoint); (F) grade 2 to 4 acute GVHD; (G) acute GI GVHD; and (H) chronic GVHD (panels F-H: secondary endpoints). Abbreviations: GI, gastrointestinal; GVHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplantation.
Recipients of vitamin A have lower cumulative incidence of graft-versus-host disease compared to placebo
Intent-to-treat analyses: cumulative incidence of (A) grade 1 to 4 acute GVHD (primary endpoint); (B) grade 2 to 4 acute GVHD; (C) acute GI GVHD; and (D) chronic GVHD (panels B-D: secondary endpoints). As-treated analyses: cumulative incidence of (E) grade 1 to 4 acute GVHD (primary endpoint); (F) grade 2 to 4 acute GVHD; (G) acute GI GVHD; and (H) chronic GVHD (panels F-H: secondary endpoints). Abbreviations: GI, gastrointestinal; GVHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplantation.
Vitamin A is thought to modulate dendritic cells to produce lower proinflammatory cytokines, which is supported by the correlative study results demonstrating lower plasma interleukin (IL)-6 (p=0.04) and plasma IL-8 (p=0.03) levels in recipients of vitamin A compared to placebo at day 14 post-HSCT. Suppression of tumorigenicity-2 (ST-2), known to be associated with acute GVHD,10 was also lower in the vitamin A arm at both day 21 post-HSCT (p=0.016) and day 100 post-HSCT (p=0.02). Median absolute CCR9+ CD8+ effector memory T (TEM) cells at day 30 post-HSCT were lower in the vitamin A arm (p=0.01), likely representing a reduction in the subset of T cells trafficking to the intestinal tract.
Correlative studies also supported the beneficial role of vitamin A in the gut microbiome and metabolome,11 as evidenced by the lower fecal dimethylamine at day 14 post-HSCT (p=0.03), as well as increased fecal short chain fatty acids (SCFAs; p=0.034) and fecal propionate (p=0.013) from days 14 to 28 post-HSCT in the vitamin A arm. Dimethylamine could be a precursor to trimethylamine, higher levels of which have been associated with GVHD.12 Higher SCFA may reflect an overall state of optimal gut health and presence of beneficial gut bacteria, leading to lower inflammation.13 Serum amyloid A1 (SAA1),14 an important vitamin A transport protein that is pro-inflammatory and implicated in inflammatory bowel disease,15 was also lower at day 21 post-HSCT in the vitamin A arm (p=0.003). To explore this finding further, SAA1 levels were investigated in an independent sample set among transplant patients with GI GVHD (n=21) and transplant patients without GVHD (n=19) and found to be strikingly elevated in GVHD patients compared to the non-GVHD controls (p=0.001).
In Brief
This is the first study to prospectively investigate the use of high-dose vitamin A prior to HSCT conditioning, with results demonstrating that this inexpensive therapy is safe, well-tolerated, and leads to decreased incidence of chronic GVHD at one year post-HSCT. Several correlative studies supported these observations, including lower plasma inflammatory markers, rising fecal SCFAs, lower SAA1 levels, and lower CCR9+ CD8+ TEM cells in the vitamin A cohort. Further studies are needed to understand the mechanism of action. Additional studies to validate these findings are currently underway in an adult HSCT population.
Disclosure Statement
Dr. Khandelwal has reported consulting activity for Incyte and is one of the co-authors of the paper discussed in this Diffusion article. Dr. Pommert indicated no relevant conflicts of interest.