The Case
You are referred an 18-year-old South Asian female patient for suspected hemochromatosis. During evaluation for primary amenorrhea, she was found to have a serum ferritin (SF) level of 2,200 ng/mL and a transferrin saturation (TS) value of 0.94. The patient’s complete blood count is normal, but her liver enzymes reveal an elevated alanine transaminase level of 67 IU/L and total bilirubin of 38 mmol/L. She has also recently been diagnosed with type 2 diabetes mellitus. Testing for the C282Y and H63D mutations of the HFE (homeostatic iron regulator) gene were negative. You are asked to provide advice on next steps for this patient.
The Question
How do you approach the diagnosis and management of a patient with suspected non-HFE hemochromatosis?
The Response
The very young age of presentation of this patient with biochemical and clinical evidence of iron overload is suggestive of a diagnosis of non-HFE hemochromatosis. Prompt evaluation and treatment is essential to avoid further morbidity.
Comparing and Contrasting HFE and Non-HFE Hemochromatosis
Hemochromatosis refers to a group of genetic disorders that result in iron overload through a failure of iron homeostasis caused by impairment of hepcidin’s response to increased iron stores.1,2 Most cases (80-90%), including nearly all those in individuals of European descent, are caused by mutations in the HFE gene (specifically homozygosity for the C282Y mutation).1,3,4 A smaller number of cases are associated with compound heterozygosity for the C282Y and H63D HFE mutations, though it is thought that most instances of true iron overload seen with this genotype are mediated through coexisting lifestyle or physiologic factors (e.g., alcohol use, metabolic dysfunction-associated steatohepatitis, or hepatitis C virus infection).1,3,4 Much rarer is hemochromatosis caused by mutations in iron regulatory genes other than HFE, including HJV (hemojuvelin), HAMP (hepcidin), and TFR2 (transferrin receptor 2).5,6 These three genes, all of which are involved in the regulation of hepcidin, share a similar pathophysiology to HFE-mediated disease. Mutations in SLC40A1 (ferroportin) are also implicated in iron overload, but with a separate pathophysiology and quite distinct biochemical and clinical features, such that iron overload caused by alterations in ferroportin is now generally considered a separate disease.2
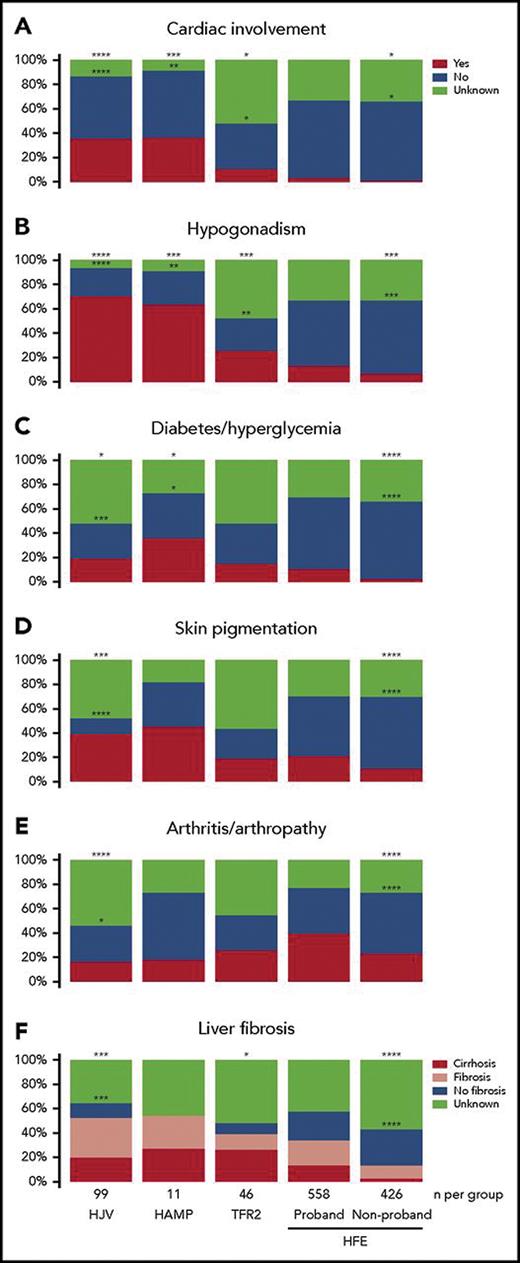
A seminal systematic review comparing published cases of patients with non-HFE hemochromatosis to a control group of those with HFE hemochromatosis from a database provided key insights into the biochemical and clinical features of these diseases.7 Figure 1 summarizes these findings; broadly speaking:
Non-HFE hemochromatosis manifests in all populations, including those of European descent, but is overall much rarer.
Non-HFE hemochromatosis has a higher penetrance and manifests clinically at an earlier age than HFE hemochromatosis. (The mean age of diagnosis is in the mid-20s for HJV- or HAMP-related disease, sometimes referred to as “juvenile hemochromatosis,” compared to the early 30s for TFR2-related disease and the early 40s for HFE-related disease).
The most severe clinical manifestations are seen in patients with HJV or HAMP mutations, with TFR2-mutated disease causing an “intermediate” severity.
While the overall clinical manifestations are similar between non-HFE and HFE-related hemochromatosis, there is greater propensity to cardiac involvement and hypogonadism in non-HFE hemochromatosis (especially in juvenile hemochromatosis).
Paradoxically, there is more arthritis and arthropathy in HFE-related hemochromatosis, and this manifestation does not appear to correlate well with the severity of iron overload.
Clinical features of patients with non-HFE and HFE-related hemochromatosis
Reproduced with permission7
Clinical features of patients with non-HFE and HFE-related hemochromatosis
Reproduced with permission7
Diagnosis of Non-HFE Hemochromatosis
The first step to diagnosing any patient with hemochromatosis is suspecting, and confirming, the presence of iron overload.1-4 Evaluation for hemochromatosis should be considered in the following circumstances:
A positive family history of confirmed hemochromatosis
The finding of elevated SF, TS, or liver enzymes in an asymptomatic patient
The presence of clinical manifestations of hemochromatosis
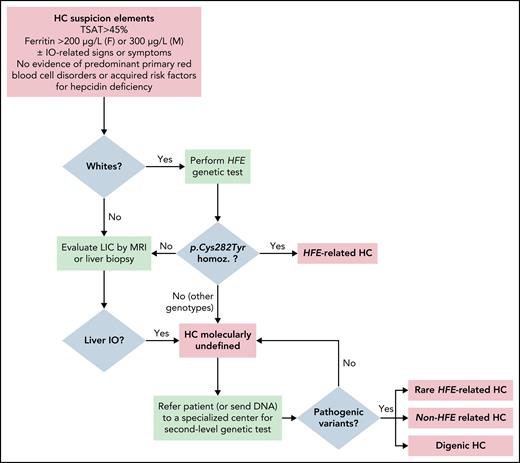
Iron overload can be reliably ruled out through simple laboratory testing. The combination of normal levels of both SF (<300ng/mL in men; <200ng/mL in women) and TS has a negative predictive value of 97% for iron overload.1,3 An isolated SF elevation with normal TS is rarely indicative of iron overload and should prompt testing for alternate causes of high SF.1,3 If hemochromatosis is still suspected, testing for HFE mutations (C282Y and H63D) should be performed, although the more pathogenic C282Y mutation is unlikely to be found in those without European ancestry, and some authors recommend omitting this step in such individuals.2-4 C282Y homozygosity is consistent with a diagnosis of HFE-related hemochromatosis, and no further testing is required.3,4 Any other combination of HFE mutations, or the lack of mutations, should prompt documentation of liver iron overload, usually done noninvasively with MRI.2-4 The finding of hepatic iron overload in the absence of secondary causes is enough to make a presumptive diagnosis of hemochromatosis. The classification scheme proposed by the International Society for the Study of Iron in Biology and Medicine provides the practical category “hemochromatosis molecularly undefined,” which at this stage allows for classification of patients where “second-level” (non-HFE) genetic testing is unavailable.2 This is very important, as lack/delay of access to the tools needed to make a specific molecular diagnosis should not delay treatment when iron overload has been confirmed and, as discussed below, would not substantially alter clinical management. However, where available, testing for non-HFE mutations (e.g., through next-generation sequencing panels) is appropriate at this point to allow for targeted testing of first-degree relatives.2 Figure 2 provides an algorithmic approach to the diagnosis of patients with hemochromatosis.
Algorithm for the diagnosis of hemochromatosis
Abbreviations: HC, hemochromatosis; IO, iron overload; LIC, liver iron concentration; TSAT, transferrin saturation.
Reproduced with permission2
Algorithm for the diagnosis of hemochromatosis
Abbreviations: HC, hemochromatosis; IO, iron overload; LIC, liver iron concentration; TSAT, transferrin saturation.
Reproduced with permission2
Management of Non-HFE Hemochromatosis
Given the rarity of non-HFE hemochromatosis, there are limited data available to provide specific management recommendations for these diseases, and clinical practice guidelines from neither the American College of Gastroenterology nor the European Association for the Study of Liver provide much comment on them.3,4 The general approach, and the approach I take, is nearly identical to that applied to patients with HFE-related hemochromatosis, with a few caveats. Given the overall high morbidity, earlier age of onset, and higher propensity for cardiac and gonadal involvement in non-HFE hemochromatosis (especially HJV- or HAMP-mediated), evaluation for both cardiac iron overload and hypogonadism is suggested in all of these patients.4 Those with abnormal liver enzymes, hepatomegaly, or SF greater than 1,000ng/mL should be referred to hepatology and undergo evaluation for hepatic fibrosis.1,3,4 First-line treatment remains therapeutic phlebotomy, with target ferritin of 50-100ng/ml, though iron chelation therapy may be considered in those with particularly severe disease, inadequate response to phlebotomy, or intolerance/contraindication to phlebotomy (e.g., congestive heart failure).3,4
Conclusion
In the patient case presented here, I suspect juvenile hemochromatosis (either HJV- or HAMP-mutated disease). I would recommend obtaining MRIs of both the liver (to document/quantify hepatic iron overload) and the heart (given the greater propensity for cardiac involvement in such patients). I would also order genetic testing (hemochromatosis genetic panels are offered through multiple commercial providers) but would not wait for the results to start treatment with phlebotomy.
Disclosure Statement
Dr. Scott has received speaker’s honoraria from Pfizer and Amgen, and travel support from Recordati Rare Diseases and Alnylam.