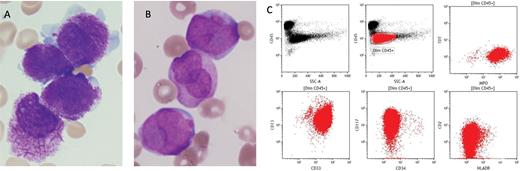
Appropriate management of acute promyelocytic leukemia (APL) relies on its prompt identification and treatment with all-trans retinoic acid (ATRA), as well as aggressive blood product support for coagulopathy.1 There are distinct morphologic features of the blasts as well as flow cytometry features that should raise suspicion for APL (Figure). Routinely used techniques to identify the genetic hallmark of APL (fusion of the RARA gene on chromosome 17q21.2, with nuclear regulatory factor gene PML on 15q24.1) include fluorescence in situ hybridization (FISH), conventional karyotype, and quantitative reverse transcription polymerase chain reaction.
Example morphology and immunophenotype of acute promyelocytic leukemia
(A) Blasts in the typical variant of acute promyelocytic leukemia (APL) showing prominent cytoplasmic granules and multiple Auer rods. (B) Blasts in the microgranular variant of APL with bi-lobed or “butterfly-shaped” nuclei. (C) By flow cytometry, the blasts in APL typically show increased side-scatter and are positive for CD117 but negative for CD34 and HLA-DR.
Example morphology and immunophenotype of acute promyelocytic leukemia
(A) Blasts in the typical variant of acute promyelocytic leukemia (APL) showing prominent cytoplasmic granules and multiple Auer rods. (B) Blasts in the microgranular variant of APL with bi-lobed or “butterfly-shaped” nuclei. (C) By flow cytometry, the blasts in APL typically show increased side-scatter and are positive for CD117 but negative for CD34 and HLA-DR.
Clustered regularly interspaced short palindromic repeats (CRISPR) technology uses the natural ability of CRISPR RNA (crRNA) to guide CRISPR-associated (Cas) enzymes to recognize and cleave nucleic acid targets (specific DNA or RNA sequence). CRISPR-Cas systems have been used for the targeted editing of genomes, epigenomes, and transcriptomes; the bioimaging of nucleic acids; the recording of cellular events; and the detection of nucleic acids.2 CRISPR-associated protein 12 (Cas12) and Cas13 enzymes have been used in the detection of viruses, bacteria, and single-nucleotide polymorphisms in TP53 and BRCA genes.2,3 As described in a 2024 Blood article,4 a CRISPR-based RNA-fusion transcript detection platform was developed for the bcr1 and bcr3 isoforms of PML::RARA (found in the majority of patients with APL) and for the two common transcript isoforms of BCR::ABL1 p210 (found in the majority of patients with chronic myeloid leukemia). For assay readout, the study used SHERLOCK (specific high-sensitivity enzymatic reporter unlocking), combining isothermal reverse transcription and recombinase polymerase amplification of target RNA with programmable Cas13-mediated detection by cleavage of a collateral reporter.4
In the proof-of-concept study by Akash Maity, PhD, and colleagues, the authors present DETECTR (DNA endonuclease-targeted CRISPR trans reporter), an assay that uses Cas12a to detect bcr1, bcr2, and bcr3 isoforms of PML::RARA. Their redefined APL identification (RAPID)-CRISPR assay includes selective amplification of bcr1, bcr2, and bcr3 via PML::RARA fusion-specific, loop-mediated isothermal amplification primers, with a single nucleotide change in the backward internal primers to create a synthetic motif site for LbCas12a recognition. Cleavage of the crRNA recognition site by LbCas12a results in collateral cleavage of a reporter molecule that can be detected by fluorescence readout or lateral flow readout. The authors used cell lines to show high fluorescence release in NB4 (PML::RARA, bcr1 isoform) and UF-1 (PML::RARA, bcr3 isoform), with only background signal observed in the control cell line (non-APL acute myeloid leukemia cells, MOLM-13). Using patient samples, the authors showed similar signal intensity among the three fusion transcript isoforms, as well as between positive peripheral blood and positive bone marrow specimens. A fluorescence cutoff was identified to discriminate PML::RARA-positive cases (66 patients) from controls (33 patients with non-APL leukemias and 10 healthy individuals), and this threshold was confirmed in a validation set of 18 samples (nine APL leukemias and nine controls). The lateral flow strip-based readout was confirmed with five APLs and five controls.
In Brief
The RAPID-CRISPR assay using Cas12a is a new tool to identify PML::RARA fusion transcripts. The authors propose that this diagnostic assay has the advantage of rapid turnaround, with the ability for results in less than three hours. This represents minimal improvement over current rapid FISH assays that can be performed in a matter of hours. Additionally, it is unnecessary to wait for genetic confirmation of APL prior to initiating ATRA, as there is very little or no toxicity from several days of ATRA exposure if the diagnosis is not APL.1 As another proposed advantage, the authors suggest the RAPID-CRISPR assay is promising for resource-limited settings, as it can be implemented in a point-of-care (POC) diagnostic setting. Successful implementation of POC testing also depends on appropriate infrastructure and trained personnel, which may be difficult to maintain for an uncommonly used assay. Improving APL outcomes in developing countries is a multifaceted process including, but not limited to, rapid diagnosis.5
Disclosure Statement
Dr. Courville indicated no relevant conflicts of interest.