The Case
A 59-year-old man underwent emergency coronary artery bypass grafting and mitral valve replacement following an acute myocardial infarction with and failed stenting due to coronary artery dissection, with a long cardiopulmonary bypass (CPB) time of 5.5 hours. Tranexamic acid was administered as a 1 g load initially, and then 1 g infusion for the case. The patient continued to bleed after protamine reversal of unfractionated heparin and was empirically transfused two units of pheresed platelets, two units of plasma, and four packed red blood cells. Following chest closure and intensive care unit (ICU) transfer, he continued to bleed more than 200 mL per hour, with coagulation tests sent on ICU arrival.
The Question
What viscoelastic tests should be ordered for surgical bleeding, and how are they used to guide therapy?
Overview of Viscoelastic Testing
Viscoelastic testing (VET), also known as viscoelastic hemostatic assay (VHA) testing, comprises whole blood coagulation assays including three major point-of-care testing systems that are commonly maintained in U.S. clinical laboratories: thromboelastography (TEG), rotational thromboelastometry (ROTEM), and sonic estimation of elasticity via resonance (SEER) sonorheometry.1,2 VET is used extensively in the perioperative setting for bleeding management during trauma, cardiac surgery, liver transplantation, obstetrical hemorrhage, and postoperative ICU management, as it provides a rapid evaluation of whole blood hemostasis (from initial clot formation to clot strength and lysis) using specific reagents.1,2
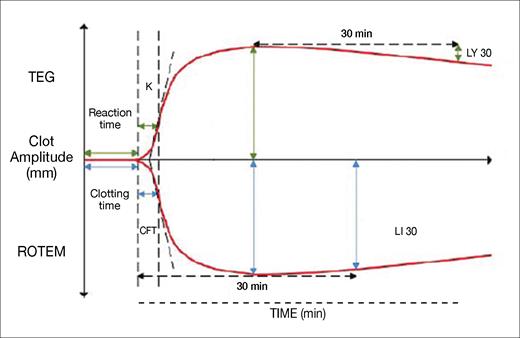
Normal TEG and ROTEM tracings are shown in Figure 1. Both testing systems provide similar information about clot formation and degradation, despite using different methodologies and terminologies. The tests measure four stages of clotting: initiation, formation, stability, and degeneration. Together, these VHAs provide clinicians with a detailed view of clot dynamics, guiding the management of coagulation disorders.
Representation of the variables obtained from a thromboelastography and rotational thromboelastometry trace
Abbreviations: CFT, clot formation time; K, clot formation time; LI 30, lysis index at 30 minutes; LY 30, lysis at 30 minutes; ROTEM, rotational thromboelastometry; TEG, thromboelastography.
Adapted from: Smith NW, Pritchett C. Point-of-care coagulation testing: viscoelastic haemostatic assays. World Federation of Societies of Anesthesiologists. November 30, 2021. resources.wfsahq.org/atotw/point-of-care-coagulation-testing-viscoelastic-haemostatic-assays
Representation of the variables obtained from a thromboelastography and rotational thromboelastometry trace
Abbreviations: CFT, clot formation time; K, clot formation time; LI 30, lysis index at 30 minutes; LY 30, lysis at 30 minutes; ROTEM, rotational thromboelastometry; TEG, thromboelastography.
Adapted from: Smith NW, Pritchett C. Point-of-care coagulation testing: viscoelastic haemostatic assays. World Federation of Societies of Anesthesiologists. November 30, 2021. resources.wfsahq.org/atotw/point-of-care-coagulation-testing-viscoelastic-haemostatic-assays
Clot initiation, recorded as “reaction time” in TEG and “clotting time” in ROTEM, refers to the time taken for the first fibrin polymerization to occur. Prolonged initiation suggests clotting factor deficiencies or the use of inhibitors like heparin, while rapid initiation may indicate early disseminated intravascular coagulation (DIC).
Clot formation, measured by clot formation time (abbreviated as K in TEG and CFT in ROTEM) and the alpha angle (not shown), depends primarily on fibrinogen polymerization and platelet aggregation. Prolonged clot formation can indicate hypofibrinogenemia or thrombocytopenia, while rapid formation suggests hyperfibrinogenemia or early DIC. The alpha angle is commonly used in research studies but not in practical clinical decision-making.
Clot stability is measured by maximum amplitude (MA) in TEG and maximum clot firmness (MCF) in ROTEM, reflecting platelet number, function, and fibrin strength. Reduced stability points to impaired platelet function, thrombocytopenia, or hypofibrinogenemia, while excessive stability suggests thrombocytosis or hyperfibrinogenemia. As thrombin is generated in the sample, platelets will be activated despite aspirin or P2Y12 blocking effects.
Clot degeneration, which represents fibrinolysis or clot breakdown, may be accelerated in hyperfibrinolysis states, such as tissue plasminogen activator release and/or major tissue injury. Clot degeneration is measured by lysis at 30 minutes post-MA (LY 30) in TEG and lysis index at 30 minutes post-MCF (LI 30) in ROTEM.
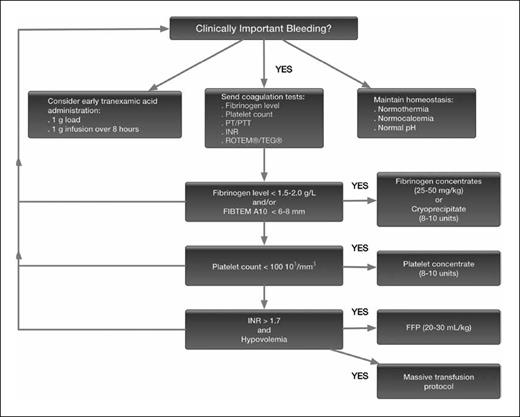
VET is used in this setting to manage bleeding, commonly targeting specific values as part of a representative algorithm, as shown in Figure 2.3 Of note, the algorithm begins with an assessment of the presence of “clinically important bleeding.” Following major surgery, particularly cardiac surgery and CPB, patients have abnormal coagulation when tested yet may not consistently be bleeding. The other benefit of VET is that if a patient’s tracing is relatively normal, suggesting functional hemostasis, the cause of the bleeding is likely a surgical bleed, which encourages surgeons to reexplore the patient. Multiple algorithms are reported, especially for cardiac surgical patients, as they commonly receive blood products in hospitals.4,5 An additional finding is that allogeneic blood use decreases and improves outcomes when using algorithms for management.6
Bleeding management algorithm
This algorithm is a suggested management strategy for the bleeding patient, with a focus on fibrinogen measurement and repletion strategies.3 Abbreviations: FFP, fresh frozen plasma; INR, international normalized ratio; PT, prothrombin time; PTT, partial thromboplastin time; ROTEM, rotational thromboelastometry; TEG, thromboelastography.
Bleeding management algorithm
This algorithm is a suggested management strategy for the bleeding patient, with a focus on fibrinogen measurement and repletion strategies.3 Abbreviations: FFP, fresh frozen plasma; INR, international normalized ratio; PT, prothrombin time; PTT, partial thromboplastin time; ROTEM, rotational thromboelastometry; TEG, thromboelastography.
The Response
Standard coagulation tests were ordered for this patient, including fibrinogen level, hemoglobin (Hb), hematocrit (Hct), platelet count, ExTEM (tissue factor activation), and FibTEM (tissue factor activation and platelet inhibition). The test results came back as follows: fibrinogen of 140 mg/dL; Hb of 8.5 g/dL; Hct of 28%; platelet count of 122,000 x 10³/µL; ExTEM clot time of 102 seconds; MCF of 42 mm; and LY 30 of 100%.
Due to persistent bleeding, multiple therapies were administered, with the patient first receiving 2 g of fibrinogen concentrate for low fibrinogen, low FibTEM, and decreased MCF. Fibrinogen replacement for bleeding management is based on either VET results or plasma levels. The transfusion threshold for fibrinogen administration in bleeding patients is typically less than 1.5 to 2g/L,7 or using a FibTEM MCF trigger level of less than 6 to 8 mm. Given these general correlations, a 140 mg/dL fibrinogen level might correspond to a FibTEM value of approximately 5 to 7 mm. However, exact correlations vary based on patient-specific factors. Of note is that factor XIII (FXIII) also contributes to the FibTEM MCF. In the U.S., cryoprecipitate is often used for fibrinogen repletion, although fibrinogen concentrates are increasingly used as in this case and have recently been approved for treating acquired fibrinogen deficiency. Many countries use fibrinogen concentrates as cryoprecipitate has been removed from use as multi-donor product in favor of specific factor concentrates.8,9 Importantly, unlike fibrinogen concentrate, cryoprecipitate also contains FXIII, von Willebrand factor, factor VIII, and procoagulant microparticles.
The patient subsequently received 1,000 units of prothrombin complex concentrate (PCC) for increased clot time and continued bleeding after fibrinogen administration. For VET, a clot time is similar to a prothrombin time using the ROTEM ExTEM, as tissue factor is the reagent used for activation. However, reaction times for TEG evaluate contact activation and are similar to the clot time for ROTEM InTEM. In a bleeding patient, plasma and PCCs are used for bleeding management and factor repletion based on VET, as shown in Figure 2, as part of “hemostatic resuscitation.” PCCs are increasingly used for bleeding management in a perioperative setting.10 Prior reports note clotting time for ExTEM correlating with prothrombin times, with reference ranges of 38 to 79 seconds for normal values. Because of persistent bleeding, PCCs were administered using approximately 12.5 IU/kg in this setting.11 Plasma may be preferred for large-volume blood loss with hypovolemia; however, PCCs in either setting are important to restore coagulation factor levels to facilitate thrombin generation if the patient is bleeding and/or coagulopathic. Hypofibrinogenemia can influence clotting times in ExTEM assays, with guidelines suggesting the importance of correcting fibrinogen levels if less than 150 mg/dL (1.5 g/l), or if ExTEM is prolonged or remains prolonged after fibrinogen replacement.11
In this case, tranexamic acid was infused for the surgery, and the LY 30 was normal on ICU arrival. Typically, patients undergoing cardiothoracic surgery receive tranexamic acid to prevent bleeding, as there is extensive data on its use to reduce transfusions.12 In VET, 30 minutes after initiation, clot lysis is determined by the clot firmness ratio to either MCF or MA. However, following trauma or liver surgery, antifibrinolytic therapy is often administered only with evidence of VET fibrinolysis due to concerns about the potential of “fibrinolytic shutdown” that impairs physiologic fibrinolysis and is frequently considered for outcomes and therapy.12
Role of Red Blood Cells in VET
The importance of red blood cells (RBCs) in clot formation is often neglected in bleeding patients, especially postoperatively. In this setting, clinicians limit RBC use but frequently generously administer allogenic blood products for bleeding management. RBCs can initiate hemostasis, with platelets stabilizing the clot structure, promoting the unfolding of the von Willebrand factor and fibrin binding. Riitta Lassila, MD, and colleagues noted that clot contraction compresses RBCs, forming polyhedrocytes to increase impermeable seals and contribute to clot mechanical strength as a scaffold supporting fibrin to increase clot strength, stability, and fibrin clot formation.13,14 However, there is no specific target hemoglobin based on bleeding management.
Conclusion
VET continues to evolve as a useful, rapid, point-of-care test that is increasingly used for goal-based therapy and patient management of major bleeding in a perioperative setting and critically ill patients. Multiple reviews and an extensive database exist regarding its use with clinically focused algorithms to guide bleeding management.15-17 Newer assays are under development for additional tests that can measure residual anticoagulation concentrations in whole blood within five to 10 minutes at the bedside and provide important clinical management strategies when major bleeding occurs.
Disclosure Statement
Dr. Levy serves on research, data safety, or advisory committees for Bayer, Grifols, Octapharma, Takeda, and Werfen. Dr. Welsby serves on advisory committees for Cerus.