In the most recent Children’s Oncology Group clinical trials, outcomes for patients with newly diagnosed childhood T-cell acute lymphoblastic leukemia (T-ALL) and lymphoblastic lymphoma (T-LL) have improved with the addition of nelarabine and bortezomib, respectively.1-4 Unfortunately, outcomes are dismal for the 10% to 25% of T-ALL and T-LL patients with relapsed/refractory (R/R) disease, with survival rates of 10% to 30% using chemotherapy regimens associated with high rates of toxicities.5,6 Nelarabine and bortezomib were previously evaluated in R/R pediatric T-ALL and T-LL, with overall response rates ranging from 38% to 68% (Table).7-9 While immunotherapeutic agents such as blinatumomab10,11 and inotuzumab12,13 have revolutionized the treatment of newly diagnosed and R/R B-cell ALL, the same has not been true for T-cell disease, due in part to the lack of available immune targets. CD38 is known to be highly expressed on T lymphoblasts, and daratumumab (DARA) — a human immunoglobulin GK monoclonal antibody targeting CD38 and approved in multiple myeloma — has demonstrated strong preclinical activity in T-ALL, prompting excitement for the introduction of immunotherapy in both T-ALL and T-LL.14,15
Responses and outcomes of recent relapsed/refractory pediatric T-cell lymphoblastic leukemia and lymphoma clinical trials
| . | DELPHINUS . | Nelarabine monotherapy7 . | NECTAR T2008-0028 . | Bortezomib AALL07P19 . |
|---|---|---|---|---|
| NCT | NCT03384654 | NCT00002970 | NCT00981799 | NCT00873093 |
| Eligibility | 1-30 years old at enrollment | <21 years old at time of diagnosis with R/R T-ALL and T-LL | 1-21 years old at enrollment | 1-31 years old at enrollment |
| Patients (T-ALL/T-LL) | T-ALL / T-LL in first relapse/refractory | T-ALL / T-LL in first relapse/refractory | T-ALL / T-LL in first relapse/refractory | T-ALL / T-LL in first relapse |
| Evaluable patients (T-ALL/T-LL) | 29 / 10 | 33 / 22 | 13 / 10 | 22 / 10 |
| CR+PR rate (T-ALL/T-LL) | 83% / 50% | 55% / 14% | 33% / 44% | 68% / 60% |
| CR rate (T-ALL/T-LL) | 52% / 40% | 48% / 0% | 17% / 33% | 68% / 30% |
| MRD negative after cycle 1 | 41% | * | * | 20% |
| Transplant rate | 72% | * | 53% | * |
| 24-month EFS (T-ALL/T-LL) | 33% / 20% | * | * | 50% |
| 24-month OS (T-ALL/T-LL) | 39% / 20% | * | * | 50% |
| . | DELPHINUS . | Nelarabine monotherapy7 . | NECTAR T2008-0028 . | Bortezomib AALL07P19 . |
|---|---|---|---|---|
| NCT | NCT03384654 | NCT00002970 | NCT00981799 | NCT00873093 |
| Eligibility | 1-30 years old at enrollment | <21 years old at time of diagnosis with R/R T-ALL and T-LL | 1-21 years old at enrollment | 1-31 years old at enrollment |
| Patients (T-ALL/T-LL) | T-ALL / T-LL in first relapse/refractory | T-ALL / T-LL in first relapse/refractory | T-ALL / T-LL in first relapse/refractory | T-ALL / T-LL in first relapse |
| Evaluable patients (T-ALL/T-LL) | 29 / 10 | 33 / 22 | 13 / 10 | 22 / 10 |
| CR+PR rate (T-ALL/T-LL) | 83% / 50% | 55% / 14% | 33% / 44% | 68% / 60% |
| CR rate (T-ALL/T-LL) | 52% / 40% | 48% / 0% | 17% / 33% | 68% / 30% |
| MRD negative after cycle 1 | 41% | * | * | 20% |
| Transplant rate | 72% | * | 53% | * |
| 24-month EFS (T-ALL/T-LL) | 33% / 20% | * | * | 50% |
| 24-month OS (T-ALL/T-LL) | 39% / 20% | * | * | 50% |
Data not provided. Abbreviations: CR, complete response; EFS, event-free survival; MRD, minimal residual disease; NCT, National Clinical Trial; OS, overall survival; PR, partial response; T-ALL, T-cell acute lymphoblastic leukemia; T-LL, t-cell lymphoblastic lymphoma.
The DELPHINUS study evaluated the addition of DARA to chemotherapy in patients aged 1 to 30 years with B-ALL after two or more relapses or T-ALL/T-LL in first relapse or refractory to one induction/consolidation regimen. Patients were required to have 5% or more bone marrow blasts or biopsy-proven lymphoma with radiologically measurable disease. Unfortunately, there were no responses in the seven B-ALL patients, and this cohort was closed due to futility. In those patients with T-ALL and T-LL, DARA was administered during cycle 1 at the approved dosing for multiple myeloma (16 mg/kg intravenous weekly on days 1, 8, 15, and 22) in combination with prednisone, vincristine, doxorubicin, and PEG-asparaginase. Identical DARA dosing was used in an optional cycle 2 in combination with cyclophosphamide, cytarabine, 6-mercaptopurine, and high-dose methotrexate. The T-cell cohort included 24 children with T-ALL, five young adults with T-ALL, and 10 patients with T-LL. While all patients received cycle 1 treatment, 76% of T-ALL (pooled) and 60% of T-LL patients also received cycle 2 treatment to achieve complete response (CR) or for consolidation prior to hematopoietic stem cell transplant (HSCT).
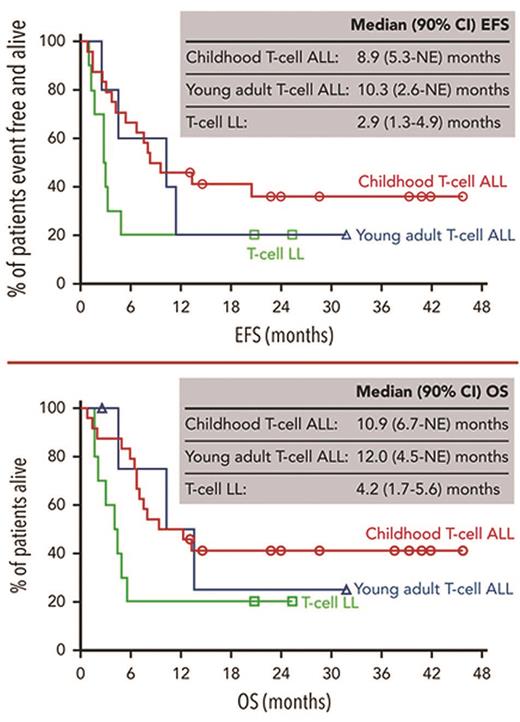
Of all T-ALL and T-LL patients, 52% and 40%, respectively, achieved CR by the end of cycle 2. These results compared favorably with the best-known response rates in prior R/R T-cell disease trials (Table), though the data are difficult to directly compare given patient heterogeneity and study-specific differences. Among DELPHINUS patients, minimal residual disease negativity rates (<0.01%) were encouraging and included 80% of those achieving CR (41% of all patients with T-ALL). Importantly, 72% of patients with T-ALL and 30% of those with T-LL were able to successfully undergo HSCT, which is recommended for patients with R/R T-ALL and T-LL based on poor outcomes.5,6 Transplant patients experienced rapid engraftment (median time to engraftment of 19 days in T-ALL and 20 days in T-LL, range 10-34 days) and a 92% hematopoietic reconstitution rate despite previous associations with delayed engraftment following DARA.16 Observed 24-month event-free survival rates were 33% and 20% for T-ALL and T-LL, respectively, while observed 24-month overall survival rates were 39% and 20%, respectively (Figure).
Event-free and overall survival of childhood and young adult T-cell lymphoblastic leukemia and lymphoma patients treated with chemotherapy and daratumumab
Abbreviations: ALL, acute lymphoblastic leukemia; EFS, event-free survival; LL, lymphoblastic lymphoma; NE, not evaluable; OS, overall survival.
Event-free and overall survival of childhood and young adult T-cell lymphoblastic leukemia and lymphoma patients treated with chemotherapy and daratumumab
Abbreviations: ALL, acute lymphoblastic leukemia; EFS, event-free survival; LL, lymphoblastic lymphoma; NE, not evaluable; OS, overall survival.
While CD38 was universally expressed in all evaluable patients with T-ALL and T-LL, those responding tended to have a higher CD38 expression at baseline compared to non-responders. Exposure to DARA resulted in reduced CD38 expression, which persisted for the duration of treatment. Serious treatment-emergent adverse events occurred in 69% and 70% of patients with T-ALL and T-LL, respectively, and were considered unrelated to DARA. While infusion-related reactions occurred in 69% of patients with T-ALL and 80% of those with T-LL, most were grade 1/2 in severity, with one grade 3 case each of abdominal pain and bronchospasm, neither of which resulted in DARA discontinuation. Importantly, none of the 36 evaluable patients developed anti-DARA antibodies, and patients were able to achieve DARA peak concentrations similar to those seen in multiple myeloma, indicating sufficient dosing in this pediatric and young adult population.
These results demonstrate that DARA added to a chemotherapy backbone is well-tolerated and effective at improving responses in children and young adults with R/R T-ALL and T-LL. This successful incorporation of immunotherapy is an exciting step forward for this high-risk and difficult-to-treat population.
In Brief
Pediatric and young adult patients with R/R T-ALL and T-LL have historically poor salvage rates and survival outcomes with traditional chemotherapy. This is the first prospective, multi-institutional study incorporating immunotherapy with a chemotherapy backbone for such patients, and the results are promising. The addition of DARA, a monoclonal antibody targeting CD38, was well-tolerated and effective in children and young adults with R/R T-ALL and T-LL, resulting in some of the highest response and survival rates seen in this patient population. Future studies including the use of DARA in upfront T-ALL and T-LL therapy are currently being planned in the pediatric and young adult population.
Disclosure Statement
The authors indicated no relevant conflicts of interest.