In recent years, technological advancements have created new opportunities in hematology diagnostics. High-parameter cytometry methods have transformed the way we interrogate the spectrum of hematopoietic and immune cells, yet these possibilities have only begun to be exploited in platelets. These techniques can provide comprehensive functional phenotyping of platelets, offering valuable insights into disease mechanisms, and they have the potential for clinical applications. However, practical challenges, including cost, data interpretation, and standardization, must be addressed before integration into routine clinical practice.
High-Dimensional Approaches in Platelet Evaluation
Conventional clinical laboratory evaluation for platelet disorders generally relies on platelet counts and functional testing such as aggregometry and granule release assays. In particular, platelet aggregation responses have long been one of the most commonly used methods for evaluating bleeding risk and diagnosing inherited platelet disorders. However, these techniques are limited by their specificity and number of parameters evaluated, and a high proportion of bleeding patients remain without a diagnosis even after comprehensive laboratory testing.1,2 Advancements in single-cell analysis have provided useful tools, such as mass cytometry and spectral (full-spectrum) flow cytometry, that enable more comprehensive phenotypic profiling.3,4
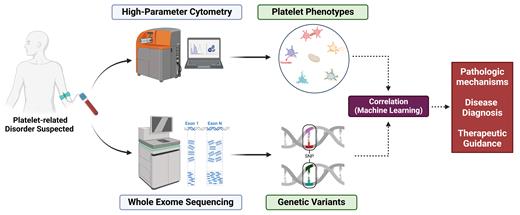
Our lab has been employing a multimodality approach, combining mass cytometry with genetic analysis, to evaluate patients with unexplained bleeding tendencies (Figure).5,6 Simultaneous evaluation of platelet constitutive and functional markers identified direct associations with genetic variants in several patients, providing valuable phenotypic insights into the pathogenicity of these variants. Multivariate and machine learning analysis identified variables most predictive of bleeding risk.6 Further investigations involving larger patient cohorts are necessary to validate and expand upon these initial correlations as well as to explore their clinical utility in diagnosing inherited platelet disorders, stratifying bleeding risk, and guiding management. Such efforts can improve patient care and also advance our understanding of the complex interplay between platelet functional phenotypes and genetic determinants.7,8
Multimodality platelet analysis by high-dimensional cytometry and genetic sequencing
Multimodality platelet analysis by high-dimensional cytometry and genetic sequencing
Platelet Heterogeneity in Health and Disease
While platelets are traditionally recognized for their critical role in hemostasis and thrombosis, accumulating evidence highlight their diverse functions in immune modulation, inflammation, and various other vascular processes.9,10 Previous studies have illustrated the diversity of platelet proteins and complexity of functional pathways that facilitate these processes.11,12 A multitude of surface receptors, intracellular signaling pathways, and secreted molecules and proteins provide platelets the functional capacity to maintain vascular hemostasis. On the other hand, alterations in complex cellular processes such as mitochondrial, apoptosis, and stress response pathways can lead to platelet dysfunction or hyperfunction and promote excessive thrombotic and inflammatory responses, which have been shown to contribute to the pathogenesis and progression of a growing number of systemic and thromboinflammatory diseases.13-15 While the traditional thought is that platelets are either resting or activated, in these two mutually exclusive states, accumulation evidence indicates significant platelet heterogeneity even within individuals.16,17
Previous studies have begun to characterize platelet functional phenotypes across various age groups.18 Using high-dimensional mass cytometry, our laboratory has recently observed phenotypic subsets of chronically activated and exhausted platelets in infants with congenital heart disease that may contribute to hematologic bleeding and thrombotic complications in these patients.19,20 Others have used these approaches to assess platelet phenotypic populations in various hematologic and immune-related diseases, including idiopathic thrombocytopenic purpura, essential thrombocythemia, inherited platelet disorders, and COVID-19.21-25 Application of high-dimensional approaches to platelets can also offer deep insights into platelet heterogeneity and functional phenotypes, including activated, aggregatory, secretory, proinflammatory, procoagulant, apoptotic, dysfunctional, and other phenotypic subpopulations that may contribute to disease pathologies.
Clinical Potential
High-dimensional approaches such as mass cytometry allow detailed phenotypic profiling and facilitate the advancement toward more personalized diagnostics. The current clinical laboratory assays for evaluating the effectiveness of antiplatelet drug responses lack robustness and reliability. We have been treating patient blood samples with various antiplatelet agents and novel inhibitors to determine their effectiveness in eliminating or reducing pathological platelet subsets (data not published). These newer high-dimensional methods could potentially offer a high-throughput method in drug screening to identify the most effective antiplatelet or combination strategy as well as in monitoring antiplatelet therapies in patients. Moreover, these methods are compatible with small sample volumes, making it feasible for use in patients with low platelet counts or in special populations like neonates.
We propose to use mass cytometry as a high-throughput method for evaluation of patients with suspected platelet pathologies that will aid in the diagnostic algorithm while providing mechanistic insights to guide therapies (Figure). The future of platelet research and diagnostics is likely to be shaped by the integration of high-dimensional technologies with machine learning and artificial intelligence (AI). As large datasets from mass cytometry and genomic analysis accumulate, AI-driven tools could facilitate automated data analysis, streamline biomarker discovery, and support the development of predictive models for platelet-related disorders. Additionally, further research into the clinical relevance of identified platelet phenotypes and genetic data will help refine diagnostic criteria and inform personalized therapeutic strategies.
Limitations and Challenges
Despite its advantages, high-dimensional platelet phenotyping faces several barriers to routine clinical application. The data generated is relatively complex, often requiring specialized bioinformatics tools and expertise for interpretation. Standardized protocols and data analysis pipelines must be developed to apply findings in a meaningful clinical context. Multicenter studies will be essential for validating the reproducibility and clinical relevance of these high-dimensional approaches across different patient populations and disease states. Furthermore, current costs and the need for advanced equipment only available at specialized research centers can be prohibitive. It is hoped that as technology advances, costs will decrease, allowing for greater accessibility to a broader range of institutions.
Conclusion
High-dimensional approaches can provide hematologists with powerful tools to capture the multifaceted functions of platelets. Characterizing platelet phenotypes can provide detailed insights into pathologic mechanisms. However, the implementation of this approach in clinical settings will require optimization of protocols and a deep understanding of its data output to translate these complex datasets into actionable insights. As we gain better understanding of platelet functional phenotypes in disease, we can develop improved diagnostics and therapies for a broad spectrum of hematologic and immune diseases.
Disclosure Statement
The authors indicated no relevant conflicts of interest.