STUDY TITLE: A Randomized, Multicenter, Phase III Trial of Tacrolimus/Methotrexate/Ruxolitinib Versus Post-Transplant Cyclophosphamide/Tacrolimus/Mycophenolate Mofetil in Non-Myeloablative/Reduced Intensity Conditioning Allogeneic Peripheral Blood Stem Cell Transplantation
CLINICALTRIALS.GOV IDENTIFIER: NCT06615050
PARTICIPATING CENTERS: 20 Blood and Marrow Transplant Clinical Trials Network sites throughout the U.S.
SPONSOR: Incyte Corporation
ACCRUAL GOAL: 572 patients, including 50 participants during the ruxolitinib dose-finding run-in phase
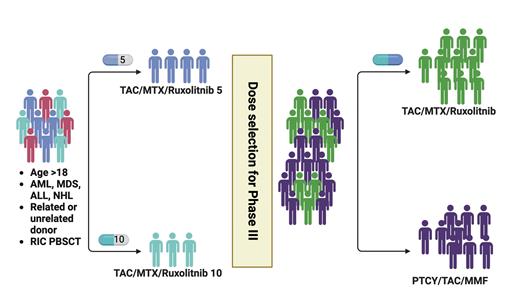
STUDY DESIGN: This multicenter, phase III, open-label, randomized trial compares two graft-versus-host disease (GVHD) prophylaxis regimens in reduced-intensity conditioning (RIC) allogeneic peripheral blood stem cell (PBSC) transplantation. The investigational regimen combines tacrolimus (TAC), mini-dose methotrexate (mini-MTX), and ruxolitinib (RUX), with RUX dosing refined during an initial run-in phase. The standard regimen uses post-transplant cyclophosphamide (PTCy), TAC, and mycophenolate mofetil (MMF). In the TAC/mini-MTX/RUX arm, TAC begins on day -3, MTX at a dose of 5 mg/m2 on days 1, 3, and 6, and RUX from day -1 through one year with taper. The PTCy/TAC/MMF arm administers a standard 50 mg/kg dose of cyclophosphamide on days 3 and 4, followed by TAC and MMF on day 5. Eligible participants include adults undergoing RIC allogeneic PBSC transplantation for myelodysplastic syndromes, acute leukemia, chronic myelomonocytic leukemia, chronic myeloid leukemia, or B- and T-cell lymphomas, with human leukocyte antigen (HLA) 7/8- or 8/8-matched sibling or unrelated donors. The primary endpoint is GVHD-free survival (GFS) at 24 months, defined as survival without grade III-IV acute GVHD, chronic GVHD requiring systemic immunosuppression, or death. Secondary outcomes include the incidence and severity of GVHD, hematologic and immune recovery, donor chimerism, relapse, non-relapse mortality, infections, graft failure, toxicity, overall and disease-free survival, and patient-reported outcomes.
RATIONALE: The rationale for this study is based on the desire for GVHD prevention methods that will be equally effective to PTCy but result in low toxicity in an older and frailer patient population. PTCy/TAC/MMF has markedly improved GVHD outcomes compared to standard TAC/MTX in RIC allogeneic transplant, reducing both acute and chronic GVHD rates.1 However, PTCy-based regimens may result in delayed engraftment, as well as increases in infection and organ toxicity in some populations. RUX, a potent and selective oral inhibitor of janus kinase (JAK)1 and JAK2, functions by modulating cytokine signaling critical to immune activation and inflammation. It has demonstrated significant activity in steroid-refractory acute and chronic GVHD, leading to U.S. Food and Drug Administration approvals for these indications. Preclinical studies showed that RUX reduces donor immune cell proliferation, suppresses harmful cytokine responses, and impairs antigen-presenting cell activity, creating a favorable immune environment for GVHD control. Prior clinical trials confirmed RUX’s ability to induce high response rates in resistant GVHD cases with manageable hematologic toxicities.2-4
Given these encouraging data, RUX’s immunemodulatory properties suggest it may be effective not only in treatment but also as a prophylactic agent to prevent GVHD. Two multicenter, phase II trials using RUX combined with TAC/mini-MTX for prophylaxis demonstrated low incidences of severe acute and chronic GVHD, with no apparent adverse impact on engraftment, and acceptable safety profiles.5,6 Additional studies have also demonstrated the feasibility and activity of RUX to improve post-transplant GVHD, with impressive overall outcomes.7-10 The biologic rationale and prior studies position RUX as a promising and nontoxic candidate for GVHD prophylaxis, particularly among older patients who may have difficulties with PTCy.
COMMENT: This phase III trial is thoughtfully designed to address the unique needs of frail patients who require RIC allogeneic PBSC transplantation but face challenges with PTCy due to toxicity. Although highly effective in reducing GVHD, PTCy can be associated with delayed engraftment and an increased risk of infections and organ toxicity, which can be particularly problematic in this vulnerable population. By combining TAC and mini-MTX with RUX, a selective JAK1/2 inhibitor known for its immunomodulatory effects, this regimen offers a potentially gentler yet efficacious alternative that may improve safety and quality of life without compromising GVHD control. A key feature of this trial is the run-in phase specifically designed to refine the optimal dosing of RUX (5 mg or 10 mg twice daily) in combination with TAC/mini-MTX. Importantly, while TAC will be tapered in both arms according to physician discretion, the protocol mandates that patients remain on RUX for a full year. This prolonged duration aims to maximize the drug’s preventative benefits by consistently modulating the inflammatory milieu during the critical post-transplant period.
The trial also incorporates pharmacologic and renal considerations related to RUX use. Because fluconazole significantly alters RUX metabolism by inhibiting CYP3A4, RUX will be dose-reduced by 50% to avoid excessive drug exposure in patients receiving concomitant fluconazole. Although posaconazole and voriconazole are also strong CYP3A4 inhibitors, the interaction between RUX and fluconazole is unique; thus, RUX dose modifications are not required with these agents. Moreover, dosing adjustments are included based on declines in creatinine clearance and in response to adverse events, including cytopenias and liver function abnormalities. These detailed guidelines demonstrate the trial’s commitment to safely integrating RUX into a prophylactic regimen for a high-risk patient population.
Much progress has been achieved in GVHD prevention in a very short period of time. This study may further advance the field through a strategy intended to reduce toxicity, while maintaining low rates of GVHD in older and frailer adults undergoing allogeneic transplantation.
Study schema
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; MDS, myelodysplastic syndromes; MMF, mycophenolate mofetil; MTX, methotrexate; NHL, non-Hodgkin lymphoma; PTCY, post-transplant cyclophosphamide; RIC PBSCT, reduced-intensity conditioning peripheral blood stem cell transplantation; TAC, tacrolimus.
Study schema
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; MDS, myelodysplastic syndromes; MMF, mycophenolate mofetil; MTX, methotrexate; NHL, non-Hodgkin lymphoma; PTCY, post-transplant cyclophosphamide; RIC PBSCT, reduced-intensity conditioning peripheral blood stem cell transplantation; TAC, tacrolimus.
Disclosure Statement
Dr. Molina indicated no relevant conflicts of interest. Dr. Muffly reports honoraria from Incyte.