Background
Both alpha- and beta-thalassemia are characterized by chronic anemia as a result of unbalanced chain synthesis, resulting in hemolysis and ineffective erythropoiesis, as well as abnormal iron hemostasis.1 While life expectancy has markedly improved as a result of transfusion therapy and iron management with chelation, morbidity from chronic transfusions, iron chelator toxicity, and chronic anemia worsens with increasing age.2,3 The decision whether to chronically transfuse ultimately rests on underlying patient characteristics such as degree of anemia, extramedullary hematopoiesis, and other factors, which dictate patient classification as transfusion-dependent thalassemia (TDT) versus non-transfusion-dependent thalassemia (NTDT). Notably, the degree of anemia (particularly hemoglobin <10 g/dL) in both TDT and NTDT may result in complications such as pulmonary hypertension, osteoporosis, extramedullary hematopoiesis, severe splenomegaly, stroke, and — ultimately — a shortened lifespan.3,4 In NTDT, a 1 g/dL increase in baseline hemoglobin is associated with a 28% reduction in morbidity risk.5 Clearly, additional therapies are needed to increase the baseline hemoglobin to mitigate these risks.
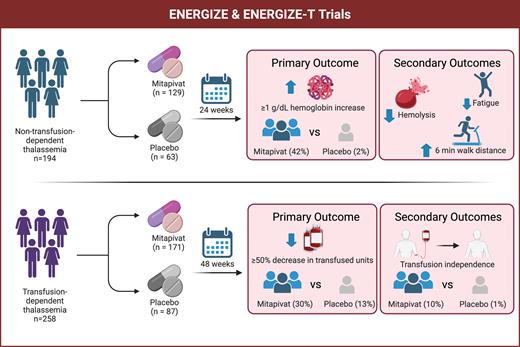
ENERGIZE and ENERGIZE-T are multinational, double-blind, randomized phase III trials of mitapivat in NTDT and TDT, respectively. Mitapivat is an oral allosteric activator of red cell-specific pyruvate kinase (PK). Activation of PK, a key enzyme that catalyzes the final irreversible step of glycolysis, increases production of adenosine triphosphate, which in turn improves red cell health and survival by preventing oxidative damage from the unpaired globin chains.6 In the phase II trial of mitapivat in NTDT, approximately 80% of participants experienced an increase in hemoglobin of 1g/dL or greater above baseline (suggesting potential therapeutic benefit), with an acceptable safety profile.7
ENERGIZE (NTDT)
Adult patients with NTDT with a baseline hemoglobin of less than 10g/dL and not taking luspatercept or other hematopoietic agents were eligible to participate in ENERGIZE (Figure). The trial included 194 participants, randomized 2:1 to 100 mg of mitapivat twice a day (n=129) or placebo (n=63) for 24 weeks. The key primary endpoint was hemoglobin response, defined as a 1 g/dL or greater increase in hemoglobin from baseline averaged over various measurements from 12 to 24 weeks. Key secondary endpoints were change in baseline Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue Scale score, a patient-reported outcome (PRO) measure for fatigue, average change in hemoglobin over 12 to 24 weeks, and distance on a six-minute walk test (6MWT).
Overview of mitapivat trials in non-transfusion-dependent and transfusion-dependent thalassemia
Created in BioRender. Wilson, S. (2025). biorender.com/mmi83sp
Overview of mitapivat trials in non-transfusion-dependent and transfusion-dependent thalassemia
Created in BioRender. Wilson, S. (2025). biorender.com/mmi83sp
As anticipated, participants in the mitapivat group were much more likely to meet the primary endpoint compared to placebo (42% vs. 2%, p<0.01). Notably, the response was observed as early as four weeks and was sustained throughout the evaluation period. Overall, the average change in hemoglobin was more modest at 0.86 g/dL (vs. -0.11 g/dL for placebo), although those who met the primary endpoint had an average increase of 1.56 g/dL. Interestingly, while markers of hemolysis such as lactate dehydrogenase, reticulocyte count, and indirect bilirubin showed improvement, erythropoietin levels remained stable at week 24.
Beyond the anticipated hematologic response, mitapivat demonstrated clinically meaningful benefits in fatigue, with a FACIT-Fatigue score change from baseline of 4.85 compared to 1.46 for placebo (a higher score is better). Additionally, mitapivat was associated with greater walking distance in the 6MWT (30.48 vs. 7.11 meters, respectively).
Overall, there were no notable safety concerns or deaths reported. Adverse events (AEs) occurred in 83% of patients on mitapivat compared to 79% on placebo, with headache (22%), initial insomnia (14%), and nausea (12%) the most commonly reported in the mitapivat group. Grade 3 or greater AEs were noted in 14% of those on mitapivat (leading to discontinuation in 3%) compared to 3% in the placebo group (with no discontinuations). Drug-induced liver injury and liver enzyme elevations were also reported with mitapivat.
ENERGIZE-T (TDT)
The companion ENERGIZE-T trial (Figure), presented in abstract form, included 258 adults with TDT who were randomized 2:1 to 100 mg of mitapivat twice a day (n=171) or placebo (n=87) — stratified by genotype — over 48 weeks. Here, the primary endpoint was transfusion reduction response (TRR), defined by a 50% or greater reduction in transfused red cells and more than two units over a consecutive 12-week period through week 48. Other endpoints assessed depths and duration of transfusion reduction and transfusion independence. The mitapivat arm was associated with a reduction in transfusion burden, with 30.4% achieving TRR compared to 12.6% of the placebo group (p<0.01). Further, 10% in the mitapivat arm achieved transfusion independence compared to 1.1% in the placebo group. Reportedly, these responses were similar across all genotype subgroups. While most participants experienced AEs regardless of arm (90% of those on mitapivat compared to 83.5% on placebo), the study reported low rates of discontinuation due to AEs (5.3% vs. 1.2%, respectively). No data on PROs such as fatigue were reported, which is unfortunate. Lastly, there was no data on iron measures, which would be expected to improve in the long term with reduced transfusions.
Limitations
Both ENERGIZE and ENERGIZE-T were limited by the overall short duration (24 vs. 48 weeks, respectively). Long-term studies of luspatercept, a competing drug with a unique mechanism of action but similar indication in beta-thalassemia, have been associated with worsening extramedullary hematopoiesis despite improvements in anemia and reduction in transfusion burden.8,9 This rare complication, which we have seen, underscores the importance of long-term pharmacovigilance. Both of these trials included an open-label extension, which will be critical in confirming long-term efficacy while capturing rare safety events. The reports of hepatocellular toxicity with mitapivat (which improved with drug discontinuation) have resulted in an update to the existing U.S. Food and Drug Administration (FDA) label and a proposal for a Risk Evaluation and Mitigation Strategy program, which could delay the time frame for regulatory approval.10
These long-term studies should also provide better insight into whether the modest improvement in anemia and transfusion burden translates to sustained, measurable clinical and quality-of-life benefits given the association of anemia and morbidity in NTDT and TDT. Specifically, the absence of a reduction in erythropoietin levels in the ENERGIZE trial brings to question whether these modest hemoglobin improvements are sufficient to modify the disease trajectory of NTDT as opposed to potential symptom improvement alone. The hemoglobin response was heterogenous yet similar across preplanned subgroups (e.g., alpha-thalassemia vs. beta-thalassemia). Further work to better predict who will respond to mitapivat would be welcome. Lastly, only adults were enrolled in these trials, and we urge the manufacturer to pursue further studies in the pediatric population, which stands to benefit more from earlier interventions.
In Brief
Together, the ENERGIZE and ENERGIZE-T trials introduce the first oral therapy for thalassemia and establish the benefit of PK activation, as mitapivat led to modest improvements in anemia, fatigue, and transfusion burden. Particular strengths of these studies are the global cohort, the inclusion of alpha-thalassemia/hemoglobin H genotypes (for which there are fewer treatment options), and the inclusion of fatigue measures for NTDT. Chronic fatigue, which can be quite morbid, is often reported in patients with NTDT, but typically insufficient to initiate transfusion therapy given the risks. FDA label expansion of mitapivat for NTDT and TDT, which we expect will be forthcoming shortly, may alter this calculus and open the door for treating more patients.
Disclosure Statement
Dr. Wilson has previously consulted with Agios Pharmaceuticals. Dr. Baskin-Miller indicated no relevant conflict of interest.