Acute myeloid leukemia (AML) remains a difficult disease to treat in both pediatric and adult populations. Furthermore, it has been repeatedly demonstrated that Black patients experience inferior survival outcomes compared with white patients.1-4 The application of pharmacogenomics to identify groups of patients that may respond differently to treatment is a growing area of interest. It was previously shown that intracellular levels of the active drug cytarabine, an essential component of AML therapy,5,6 is associated with treatment efficacy.7 Single nucleotide polymorphisms in the ara-C metabolic pathway correlate with intracellular ara-C active drug levels,8 leading to the development of the ara-C pharmacogenomics score ACS10, which consists of 10 single nucleotide variants in nine ara-C metabolic pathway genes. Low ACS10 scores are associated with poor overall survival (OS) and event-free survival (EFS) in pediatric AML patients, and low scores are more common in Black patients.9
Richard Marrero, PharmD, PhD, and colleagues recently evaluated ACS10 scores in large pediatric (n=717) and adolescent and young adult (AYA; n=369) cohorts with newly diagnosed AML, with the goal of evaluating its applicability to an AYA cohort and examining its association with race and treatment outcomes. The pediatric cohort included patients enrolled in Children’s Oncology Group AAML1031 trial arms A (n=334) and B (n=383), which randomized patients with newly diagnosed AML to receive either standard induction therapy with cytarabine, daunorubicin, and etoposide (ADE; arm A) or ADE plus bortezomib (arm B).10 The AYA cohort comprised patients enrolled in nine different upfront trials from the Alliance for Clinical Trials in Oncology, with similar intensive induction regimens. ACS10 scores were calculated for each patient based on previously published work,9 with patients divided into low ACS10 score or high ACS10 score groups.
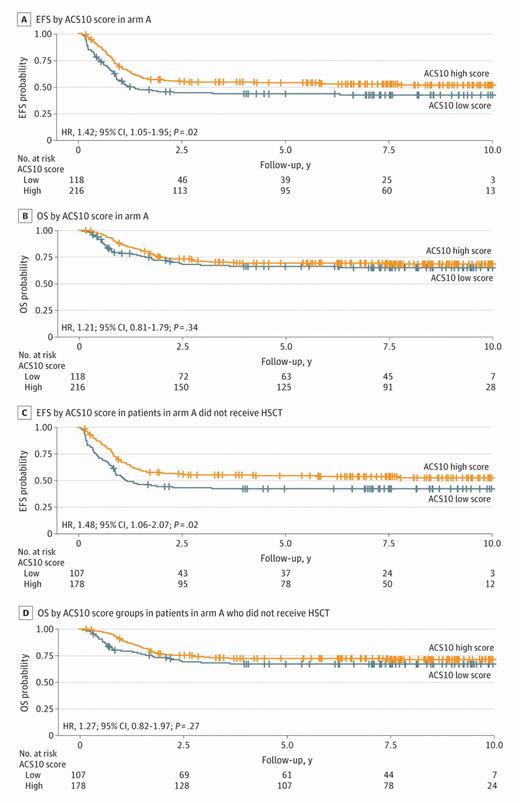
Within the pediatric cohort overall, there was no difference in EFS or OS between the low and high ACS10 score groups. However, within standard arm A, patients in the low ACS10 score group had significantly worse EFS compared with those in the high ACS10 score group (hazard ratio [HR] = 1.42 [95% CI 1.05-1.95; p=.02]; five-year EFS: 41% vs. 52%). This difference in EFS was not observed within experimental arm B, validating previous findings that the negative impact of a low ACS10 score can be overcome with therapy augmentation in pediatrics,9 in this case with the addition of bortezomib.
Similar trends were identified within the AYA cohort. Among all AYA patients analyzed, those with a low ACS10 score had a higher point estimate for OS and EFS compared with those in the high ACS10 score group. Among patients who did not receive hematopoietic stem cell transplant, those with a low ACS10 score had significantly inferior OS compared with those with a high ACS10 score (HR=1.50 [95% CI 1.05-2.14; p=.03]; three-year OS rates: 38% [95% CI 27%-49%] vs. 55% [95% CI 47%-62%]). Importantly, this analysis is the first to evaluate ACS10 scores and outcomes outside of the pediatric population.
In line with their previous study,9 in both cohorts, more Black patients than white patients had low ACS10 scores. Within the pediatric AAML1031 cohort, 12% of patients (n=84) identified as Black, 69% (n=58) of whom had low ACS10 scores, compared with 28% of white patients. Similarly, in the AYA Alliance cohort, 9% of patients (n=32) identified as Black, 84% (n=27) of whom had low ACS10 scores, compared with 22% of white patients. In the pediatric cohort, significantly worse EFS and OS were observed for Black patients on standard treatment (arm A); however, this difference in survival was not seen with the addition of bortezomib (arm B; Figure), suggesting that therapy augmentation with bortezomib is indeed associated with a survival benefit for Black patients. Taken together with previous findings, these results suggest that genetic differences in cytarabine metabolism may at least in part explain the differences in treatment outcomes that have long been observed between Black and white patients with AML, and these patients may benefit from augmented induction therapy.
Kaplan-Meier survival curves within each AAML1031 treatment arm by race
Event-free survival (EFS) by race in treatment arm A (A) and arm B (C); overall survival (OS) by race in treatment arm A (B) and arm B (D). White patients used as reference group. Reprinted from Marrero, et al.
Kaplan-Meier survival curves within each AAML1031 treatment arm by race
Event-free survival (EFS) by race in treatment arm A (A) and arm B (C); overall survival (OS) by race in treatment arm A (B) and arm B (D). White patients used as reference group. Reprinted from Marrero, et al.
In Brief
This analysis validates previous findings of the association between low ACS10 scores and poorer survival outcomes in pediatric patients with AML,9 as well as demonstrates its applicability to an AYA cohort. Furthermore, low ACS10 scores were found in a greater number of Black patients, demonstrating that the poorer outcomes associated with these low scores could be mitigated by therapy augmentation. This is promising groundwork for future development of personalized induction regimens based on ACS10 scores to eliminate racial disparities in AML outcomes.
Disclosure Statement
The authors indicated no relevant conflicts of interest.