In this paper, Grosser and colleagues review our understanding of the physiology and pharmacology of the cyclooxygenase (COX)/hydroperoxidase arachadonate modifying systems, the clinical trials that led to FDA approval for the first three COX-2 inhibitors, the five larger clinical trials and post-marketing data that led to the withdrawal of many of the drugs because of cardiovascular complications, and the biological explanation(s) for these events. While a complete understanding of the reasons for the excess morbidity and mortality due to the thrombogenesis, hypertension, and atherogenesis associated with COX-2 inhibitor use is not yet available, the authors carefully discuss the critical physiology, the excitement engendered by the "COX-2 hypothesis," and the unfortunately all-too-brief clinical trials leading to FDA approval of several COX-2 specific inhibitory agents. They then deliver an insightful post-hoc analysis of the pathogenesis of the complications of these agents. Their conclusion is that the cardiovascular risks of COX-2 inhibition are likely due to a confluence of undesirable effects of these agents, and that a thorough understanding of prostaglandin (PG) physiology could/should have allowed prediction of these complications.
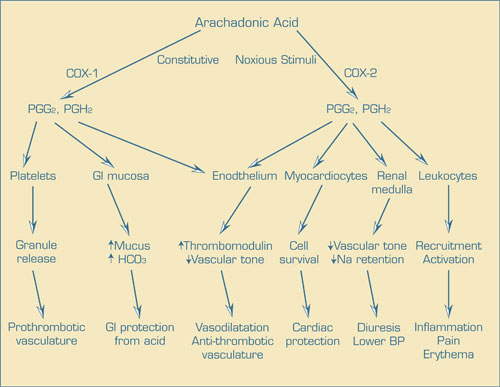
Cyclooxygenases (COX) 1 and 2 are highly-related bisfunctional enzymes that convert arachadonic acid into the PG endoperoxide intermediates PGG2 and PGH2, which are then converted to a wide array of biologically active PGs, prostacyclins (PGI), and thromboxanes (TX). The most pathologically relevant activities of these compounds are leukocyte recruitment and activation at sites of noxious stimuli (PGE2 and PGI2), events responsible for the signs and symptoms of inflammation. To combat the pain and tissue damage caused by inflammation, potent COX inhibitors (non-steroidal anti-inflammatory drugs [NSAIDs]) have found widespread use. Because another important physiological function of PGE2 is gastrointestinal mucosal protection, NSAID use is associated with significant toxicity. When it was realized that gastrointestinal mucosal PGE2 is produced primarily by COX-1, specific COX-2 inhibition became an attractive target for the pharmaceutical industry. However, a number of other physiological events are also mediated by PG, PGI, and TX, including enhancement of platelet aggregation and reactivity within the vascular wall by TxA2, maintenance of an anti-thrombotic phenotype in endothelial cells, down modulation of vascular smooth muscle tone, increased renal cortical renin response to baroreceptors, and reduced oxidative injury to cardiac myocytes by PGI2. COX-1 is constitutively expressed in most tissues, while COX2 is induced in inflammatory states. The "COX-2 hypothesis" posits that much or most of the pain and suffering of PG mediated inflammation is due to the induction of COX-2 and that the major complication of NSAIDs, GI intolerance, is due to COX-1 inhibition of PGE2 in the GI tract. Although it is well known that COX inhibition with NSAIDs blocks not only the anti-thrombotic phenotype mediated by PGI2 but also the prothrombotic effects of platelet TxA2, suggesting that COX-2 inhibition would only affect endothelial PGI2 and not platelets (which do not express COX-2) TxA2 (and give rise to a prothrombotic phenotype), it was claimed that the endothelial PG effect was redundant to vascular wall-derived nitric oxide (NO), so that untoward effects should not emerge following COX-2 inhibition. However, careful consideration of the prothrombotic phenotype of PGI2 receptor-null mice suggests that the untoward effects of COX-2 inhibition should have been better anticipated. Moreover, in the clinical realm, Grosser and colleagues critique the data used in support of drug effectiveness and safety. For example, all three safety studies were too small and underpowered to detect a significant influence on health. However, subsequent clinical studies which led to drug withdrawal demonstrated, like in several animal models, that persons who display an enhanced risk of cardiovascular morbidity (e.g., post coronary artery bypass grafting) were also at risk for the development of myocardial infarction and stroke, even in a small clinical trial of short duration. Based on their analysis, Grosser and colleagues conclude that several lessons are readily apparent in carefully considering the in vitro, pre-clinical, and clinical trial data relating to the COX-2 hypothesis; that an interdisciplinary, integrated, and real time approach to drug development is essential to avoid the silos of information that failed to inform clinical development of the COX-2 inhibitors, and that better post-marketing data collection must be established and be continuously monitored with formal clinical decision-making analyses, lest we repeat this example of an unfortunate outcome of fooling with normal physiology.
Competing Interests
Dr. Kaushansky indicated no relevant conflicts of interest.