Dr. Petruzzelli indicated no relevant conflicts of interest.
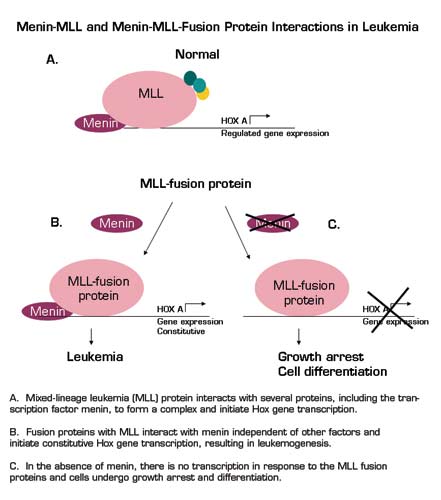
The mixed-lineage leukemia (MLL) protein is a histone methyl transferase that covalently modifies chromatin. In leukemia it is found in an array of chromosomal translocations, and its overexpression leads to enhanced Hox gene expression. This, in part, may contribute to the development of the leukemic phenotype and disruption of normal hematopoiesis. Interestingly, MLL, when it is not fused to another gene, acts by forming a complex with a set of proteins that are similar to a family of proteins that interact with histone methyl transferases in yeast; this complex is associated with a specific methylation event that results in the maintenance of gene expression. The MLL-fusion proteins that are seen in leukemia are no longer able to associate with this complex of proteins, but still result in the activation of gene transcription. In this paper, the investigators probe the molecular basis by which fusion proteins of MLL modulate gene expression and result in leukemic transformation.
Menin is known to bind to MLL, and this event is unique when compared to the highly-conserved binding partners of MLL that are shared with yeast. Menin is the protein product of Men-1, the tumor suppressor gene that is lost in tumors of heritable and sporadic endocrine disorders. Previous work demonstrated that menin still associates with the MLL-fusion protein even though other members of the complex do not. The experiments presented in this manuscript demonstrate that MLL and menin associate on Hox gene promoters. The proteins interact through specific motifs in the MLL portion of the fusion protein, and when specific regions are mutated, menin-MLL association is lost and cellular transformation is disrupted. The need for continued expression of menin to initiate and maintain transformation was specific for MLL - other fusion proteins that induce transformation did not have this requirement. Disruption of the interaction of an oncogenic fusion protein (MLL) with a tumor suppressor (menin) results in differentiation, and disrupts proliferation of leukemic blasts. Lastly, if menin expression is disrupted, Hox gene expression is diminished and cells undergo differentiation.
In Brief
This work ties together several critical observations that characterize the mechanism for the formation and maintenance of a subset of leukemias. Although MLL is known to be a methyl transferase that activates Hox genes through the interaction of a series of highly conserved proteins, the activating fusion proteins that are seen in leukemia no longer act through this large protein complex but, rather, are able to set things in motion through the interaction with a single protein, menin. This event is so potentially interesting because it not only sheds mechanistic light on the regulation of cellular growth by a fusion protein, but also may lead to unique therapeutic options in at least this subset of leukemias.
Although one has to be concerned that targeting a tumor suppressor gene known to be associated with an array of endocrine tumors may lead to trouble, the investigators were able to finely map the interaction domains and found that at least two sites on MLL were involved in menin binding. Since there are multiple sites that can potentially be targeted, the potential therapeutic options in this interaction remain broad. Furthermore, high-affinity binding through a specific site on MLL was required for cellular transformation. A reduction in affinity of the MLL-menin interaction may be sufficient to abrogate the transformed phenotype. Agents that aim to diminish affinity of these proteins for each other may be sufficient to result in loss of maintenance of the leukemia cell phenotype that appears to be dependent on the continued presence of the MLL fusion protein-menin interaction, but may not affect other key regulatory components of the cellular homolog. With this insightful manuscript, the investigators advance understanding of the connection between a fusion protein product and its effect on cellular gene expression and growth, and also expand the horizon for potential drug targets.
Competing Interests
Dr. Petruzzelli indicated no relevant conflicts of interest.