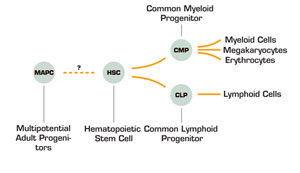
The bone marrow is a remarkable organ. Every time your heart beats, millions of cells are created. The turnover of these cells requires a tightly regulated balance between the formation of all these blood cells and their senescence and death. Our bodies are able to choreograph this birth and death routine in part due to stem cells, which differentiate and self-renew with each division. The characterization of hematopoietic stem cells has been a remarkably fruitful area of research. Our bone marrow contains at least two types of stem cells, the hematopoietic stem cell (HSC) and a stromal stem cell that is able to give rise to cartilage, adipose tissue, muscle, and bone. One area of exciting research has been the identification of multipotent adult progenitor cells (MAPCs), which could be coaxed from the bone marrow and which are thought to be the precursors for the non-hematopoietic cells. In this paper, Catherine Verfaillie’s laboratory describes convincing data demonstrating that these MAPCs can be expanded in vitro for more than 40-80 population doublings without senescence and that these cells are capable of multilineage engraftment in immunodeficient mice, although approximately 1000-fold more MAPCs were required compared to purified HSC. The HSC derived from these MAPCs are able to be serially transplanted into secondary and tertiary recipients and the lymphocytes derived from these MAPCs were fully functional. Moreover, using green fluorescent protein transgenic mice and congenic mice, the data suggests that fusion did not occur.
In Brief
One of the many holy grails in hematology is the ability to expand hematopoietic stem cells. This goal has been elusive in that when bone marrow is placed in culture, there can be a dramatic increase in committed precursors, but there seems to be a limit in the number of true HSCs that expand. Consequently, when these expanded cells are returned to the recipient, there is little beneficial clinical effect that can be observed. These MAPCs, in contrast, seem to be able to expand for prolonged periods ex vivo without evidence of senescence. The promise of these MAPCs is that they can contribute robustly to hematopoiesis, generating myeloid and lymphoid cells that can be serially transplanted. Moreover, no tumor formation was observed, which will be important in therapeutic applications. The immediate applications include expansion of HSCs for bone marrow reconstitution or the use of these cells to induce transplantation tolerance. It is not clear yet how these adult MAPCs will function compared to embryonic stem cells or whether these cells will be able to be more broadly used for regenerative medicine outside of hematopoiesis. However, for the time being, the possibility of a readily expandable population of cells that can make blood is truly exciting.
Competing Interests
Dr. Chao indicated no relevant conflicts of interest.