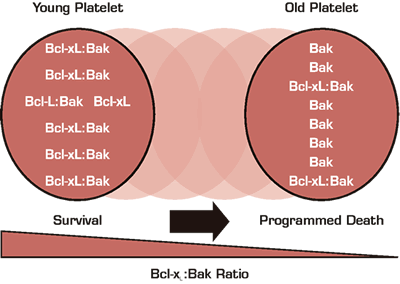
Genome-wide mutagenesis (so-called “forward genetics”) has been used widely in non-vertebral model systems (including flies, worms, yeast, and bacteria) to discover pathways involved in numerous cellular processes. Similar approaches have been developed for mouse research, but are not widely used because of the time and costs related to mouse breeding. Nevertheless, some intrepid investigators have begun to experiment with mouse forward genetics and in hematology, successes have been reported in the areas of thrombosis and innate immunity. In this manuscript, this approach was used to identify pathways involved in controlling circulating platelet number. The investigators discovered two point mutations in a single gene that were associated with dominantly inherited modest thrombocytopenia. The gene, Bcl-xL, is a “pro-survival” member of the Bcl-2 gene family that is known to regulate the mitochondrial pathway of programmed cell death. In an elegant series of studies they confirmed the discovery using a mouse strain heterozygous for a null mutation in Bcl-xL and a pharmacologic agent, ABT-737, that blocks the function of Bcl-xL. To probe the mechanism of the thrombocytopenia, the investigators showed that the mutant mice had normal bone marrow and spleen morphology and in vitro megakaryopoiesis, but dramatically shortened platelet survival; circulating platelet t½ in heterozygous and homozygous mice were respectively ~50 percent and 80 percent lower than wildtype. By transplanting Bcl-xL deficient platelets into wildtype mice, they showed that the defect was inherent to the platelet. Finally, they showed that mice deficient for Bak, a “pro-apoptotic” Bcl-2 family member known to bind to Bcl-xL, had the opposite phenotype — they were thrombocytotic with prolonged platelet survival. Importantly, Bak deficiency “rescued” the thrombocytopenic phenotype of the Bcl-xL mutants. The investigators thus propose that platelets are genetically programmed to die by Bak-mediated apoptosis. In “young” platelets this is held in check by Bcl-xL, which binds and neutralizes Bak. Since the Bcl-xL protein is less stable than Bak, as platelets age in the circulation Bcl-xL levels drop, eventually to the point where Bak inhibition is lost and cell death is triggered. In essence, Bcl-xL instability serves as a molecular clock ticking down towards the inevitable destruction of the circulating platelet.
In Brief
Recent discoveries have challenged many longstanding beliefs about platelet function and biology. For example, we now know that even though platelets do not have nuclei, they contain RNA and have the machinery to process it and translate it, and may thus contribute to inflammation and thrombosis in manners not previously appreciated. Furthermore, platelets express many of the components of the apoptotic machinery, including caspases and Bcl family members. These components have been postulated to play a role in platelet fragmentation from bone marrow megakaryocyte precursors and their presence in circulating platelets thought perhaps to be a relic from the parent megakaryoctye. It is now clear, however, that while platelets are incapable of undergoing “classic” apoptosis in that they do not have nuclei to condense or DNA to fragment, they are capable of undergoing a form of programmed cell death. In this important manuscript, the authors used a sophisticated genetic approach to discover that programmed platelet death is regulated by the balance of pro- and anti-apoptosis proteins Bak and Bcl-xL, and that this balance determines the limit on circulating platelet life span. The ability to manipulate this balance pharmacologically to extend platelet lifespan holds considerable promise for future treatment of thrombocytopenic conditions and for storage of platelets for transfusion.
Competing Interests
Dr. Silverstein indicated no relevant conflicts of interest.